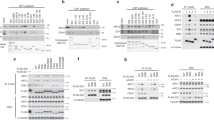
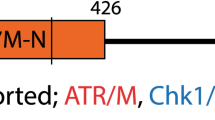
Abstract
Proper control of entry into and progression through mitosis is essential for normal cell proliferation and the maintenance of genome stability1,2,3,4. The mammalian mitotic kinase Polo-like kinase 1 (Plk1) is involved in multiple stages of mitosis5. Here we report that Forkhead Box M1 (FoxM1), a substrate of Plk1 (refs 6, 7, 8), controls a transcriptional programme that mediates Plk1-dependent regulation of cell-cycle progression. The carboxy-terminal domain of FoxM1 binds Plk1, and phosphorylation of two key residues in this domain by Cdk1 is essential for Plk1–FoxM1 interaction. Formation of the Plk1–FoxM1 complex allows for direct phosphorylation of FoxM1 by Plk1 at G2/M and the subsequent activation of FoxM1 activity, which is required for expression of key mitotic regulators, including Plk1 itself. Thus, Plk1-dependent regulation of FoxM1 activity provides a positive-feedback loop ensuring tight regulation of transcriptional networks essential for orderly mitotic progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nigg, E. A. Mitotic kinases as regulators of cell division and its checkpoints. Nature Rev. Mol. Cell Biol. 2, 21–32 (2001).
Norbury, C. & Nurse, P. Animal cell cycles and their control. Annu. Rev. Biochem. 61, 441–70 (1992).
Pines, J. & Rieder, C. L. Re-staging mitosis: a contemporary view of mitotic progression. Nature Cell Biol. 3, E3–6 (2001).
Murray, A. W. Recycling the cell cycle: cyclins revisited. Cell 116, 221–34 (2004).
Barr, F. A., Sillje, H. H. & Nigg, E. A. Polo-like kinases and the orchestration of cell division. Nature Rev. Mol. Cell Biol. 5, 429–40 (2004).
Laoukili, J. et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nature Cell Biol. 7, 126–36 (2005).
Major, M. L., Lepe, R. & Costa, R. H. Forkhead box M1B transcriptional activity requires binding of Cdk–cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol. Cell Biol. 24, 2649–61 (2004).
Wang, I. C. et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2–Cks1) ubiquitin ligase. Mol. Cell Biol. 25, 10875–94 (2005).
Anderson, M. et al. Plo1(+) regulates gene transcription at the M-G(1) interval during the fission yeast mitotic cell cycle. EMBO J. 21, 5745–55 (2002).
Cho, R. J. et al. A genome-wide transcriptional analysis of the mitotic cell cycle. Mol. Cell 2, 65–73 (1998).
Darieva, Z. et al. Polo kinase controls cell-cycle-dependent transcription by targeting a coactivator protein. Nature 444, 494–8 (2006).
Watanabe, N. et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta–TrCP. Proc. Natl Acad. Sci. USA 101, 4419–24 (2004).
Toyoshima-Morimoto, F., Taniguchi, E. & Nishida, E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 3, 341–8 (2002).
Casenghi, M. et al. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev. Cell 5, 113–25 (2003).
Hansen, D. V., Loktev, A. V., Ban, K. H. & Jackson, P. K. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFβTrCP-dependent destruction of the APC Inhibitor Emi1. Mol. Biol. Cell. 15, 5623–34 (2004).
Neef, R. et al. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863–75 (2003).
Zhou, T., Aumais, J. P., Liu, X., Yu-Lee, L. Y. & Erikson, R. L. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev. Cell 5, 127–38 (2003).
Wonsey, D. R. & Follettie, M. T. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res. 65, 5181–9 (2005).
Leung, T. W. et al. Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett. 507, 59–66 (2001).
Jang, Y. J., Lin, C. Y., Ma, S. & Erikson, R. L. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc. Natl Acad. Sci. USA 99, 1984–9 (2002).
Seong, Y. S. et al. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 277, 32282–93 (2002).
Elia, A. E. et al. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 115, 83–95 (2003).
Lowery, D. M., Lim, D. & Yaffe, M. B. Structure and function of Polo-like kinases. Oncogene 24, 248–59 (2005).
Elia, A. E., Cantley, L. C. & Yaffe, M. B. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 299, 1228–31 (2003).
Gumireddy, K. et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell 7, 275–86 (2005).
Yarm, F. R. Plk phosphorylation regulates the microtubule-stabilizing protein TCTP. Mol. Cell Biol. 22, 6209–21 (2002).
Nakajima, H., Toyoshima-Morimoto, F., Taniguchi, E. & Nishida, E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278, 25277–80 (2003).
Sherr, C. J. & McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2, 103–12 (2002).
Acknowledgements
We are grateful for extensive discussions with R.H. Medema and J. Laoukili and their input. We thank H.M. Thompson for editing the manuscript. Mass spectrometry analysis was performed by Taplin Biological Mass Spectrometry Facility at Harvard University. This work was supported in part by grants from the National Institutes of Health (NIH RO1 CA113381 to JC), Mayo SPORE P50 (CA116201 project no. 1 to J.C.) and the T.J. Martell Foundation (to D.J.T.). J.C. is a recipient of an Era of Hope Scholars award from DOD. Z.F. is a recipient of a Ruth L. Kirschstein NRSA individual Fellowship from NIH.
Author information
Authors and Affiliations
Contributions
Z.F. performed most of experiments, analysed the data and wrote the paper; L.M. and J.M.V. analysed the time-lapse imaging data; J.H. performed the experiments shown in Fig. S2a and b; W.W. and H. L. synthesized ON01910; Z.F. and J.C. designed the experiments; J.C. and D.J. T. supervised the study and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5 and Supplementary Discussion (PDF 1128 kb)
Supplementary Information
Supplementary Movie 1 (AVI 317 kb)
Supplementary Information
Supplementary Movie 2 (AVI 376 kb)
Rights and permissions
About this article
Cite this article
Fu, Z., Malureanu, L., Huang, J. et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol 10, 1076–1082 (2008). https://doi.org/10.1038/ncb1767
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1767
This article is cited by
-
A co-formulation of interferons alpha2b and gamma distinctively targets cell cycle in the glioblastoma-derived cell line U-87MG
BMC Cancer (2023)
-
Master mitotic kinases regulate viral genome delivery during papillomavirus cell entry
Nature Communications (2023)
-
Inhibition of USP7 induces p53-independent tumor growth suppression in triple-negative breast cancers by destabilizing FOXM1
Cell Death & Differentiation (2023)
-
Development of an interfering peptide M1-20 with potent anti-cancer effects by targeting FOXM1
Cell Death & Disease (2023)
-
PLK1 maintains DNA methylation and cell viability by regulating phosphorylation-dependent UHRF1 protein stability
Cell Death Discovery (2023)