Abstract
Eukaryotic genomes are partitioned into chromosomes that form compact and spatially well-separated mechanical bodies during mitosis1,2,3. This enables chromosomes to move independently of each other for segregation of precisely one copy of the genome to each of the nascent daughter cells. Despite insights into the spatial organization of mitotic chromosomes4 and the discovery of proteins at the chromosome surface3,5,6, the molecular and biophysical bases of mitotic chromosome structural individuality have remained unclear. Here we report that the proliferation marker protein Ki-67 (encoded by the MKI67 gene), a component of the mitotic chromosome periphery, prevents chromosomes from collapsing into a single chromatin mass after nuclear envelope disassembly, thus enabling independent chromosome motility and efficient interactions with the mitotic spindle. The chromosome separation function of human Ki-67 is not confined within a specific protein domain, but correlates with size and net charge of truncation mutants that apparently lack secondary structure. This suggests that Ki-67 forms a steric and electrostatic charge barrier, similar to surface-active agents (surfactants) that disperse particles or phase-separated liquid droplets in solvents. Fluorescence correlation spectroscopy showed a high surface density of Ki-67 and dual-colour labelling of both protein termini revealed an extended molecular conformation, indicating brush-like arrangements that are characteristic of polymeric surfactants. Our study thus elucidates a biomechanical role of the mitotic chromosome periphery in mammalian cells and suggests that natural proteins can function as surfactants in intracellular compartmentalization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kschonsak, M. & Haering, C. H. Shaping mitotic chromosomes: From classical concepts to molecular mechanisms. BioEssays 37, 755–766 (2015)
Hirano, T. Condensin-based chromosome organization from bacteria to vertebrates. Cell 164, 847–857 (2016)
Ohta, S., Wood, L., Bukowski-Wills, J. C., Rappsilber, J. & Earnshaw, W. C. Building mitotic chromosomes. Curr. Opin. Cell Biol. 23, 114–121 (2011)
Naumova, N. et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013)
Van Hooser, A. A., Yuh, P. & Heald, R. The perichromosomal layer. Chromosoma 114, 377–388 (2005)
Ohta, S. et al. The protein composition of mitotic chromosomes determined using multiclassifier combinatorial proteomics. Cell 142, 810–821 (2010)
Fuller, B. G. et al. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature 453, 1132–1136 (2008)
Neumann, B. et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464, 721–727 (2010)
Hériché, J. K. et al. Integration of biological data by kernels on graph nodes allows prediction of new genes involved in mitotic chromosome condensation. Mol. Biol. Cell 25, 2522–2536 (2014)
Whitfield, M. L., George, L. K., Grant, G. D. & Perou, C. M. Common markers of proliferation. Nature Rev. Cancer 6, 99–106 (2006)
Yerushalmi, R., Woods, R., Ravdin, P. M., Hayes, M. M. & Gelmon, K. A. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 11, 174–183 (2010)
Saiwaki, T., Kotera, I., Sasaki, M., Takagi, M. & Yoneda, Y. In vivo dynamics and kinetics of pKi-67: transition from a mobile to an immobile form at the onset of anaphase. Exp. Cell Res. 308, 123–134 (2005)
Verheijen, R. et al. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. II. Localization in mitotic cells and association with chromosomes. J. Cell Sci. 92, 531–540 (1989)
Booth, D. G. et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. eLife 3, e01641 (2014)
Sobecki, M. et al. The cell proliferation antigen Ki-67 organises heterochromatin. eLife 5, e13722 (2016)
Vanneste, D., Takagi, M., Imamoto, N. & Vernos, I. The role of Hklp2 in the stabilization and maintenance of spindle bipolarity. Curr. Biol. 19, 1712–1717 (2009)
Beaudouin, J., Gerlich, D., Daigle, N., Eils, R. & Ellenberg, J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell 108, 83–96 (2002)
Cidado, J. et al. Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget 7, 6281–6293 (2016)
Hofmann, K. & Bucher, P. The FHA domain: a putative nuclear signalling domain found in protein kinases and transcription factors. Trends Biochem. Sci. 20, 347–349 (1995)
Schlüter, C. et al. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol. 123, 513–522 (1993)
Takagi, M., Matsuoka, Y., Kurihara, T. & Yoneda, Y. Chmadrin: a novel Ki-67 antigen-related perichromosomal protein possibly implicated in higher order chromatin structure. J. Cell Sci. 112, 2463–2472 (1999)
Takagi, M., Sueishi, M., Saiwaki, T., Kametaka, A. & Yoneda, Y. A novel nucleolar protein, NIFK, interacts with the forkhead associated domain of Ki-67 antigen in mitosis. J. Biol. Chem. 276, 25386–25391 (2001)
Rosen, M. J. & Kunjappu, J. T. Surfactants and Interfacial Phenomena 4th edn (John Wiley & Sons, 2012)
Milner, S. T. Polymer brushes. Science 251, 905–914 (1991)
Hyman, A. A., Weber, C. A. & Jülicher, F. Liquid–liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 (2014)
Brangwynne, C. P., Mitchison, T. J. & Hyman, A. A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl Acad. Sci. USA 108, 4334–4339 (2011)
Zwicker, D., Decker, M., Jaensch, S., Hyman, A. A. & Jülicher, F. Centrosomes are autocatalytic droplets of pericentriolar material organized by centrioles. Proc. Natl Acad. Sci. USA 111, E2636–E2645 (2014)
Schmitz, M. H. & Gerlich, D. W. Automated live microscopy to study mitotic gene function in fluorescent reporter cell lines. Methods Mol. Biol. 545, 113–134 (2009)
Ran, F. A. et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 154, 1380–1389 (2013)
Guizetti, J. et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616–1620 (2011)
Lukinavičius, G. et al. SiR-Hoechst is a far-red DNA stain for live-cell nanoscopy. Nature Commun . 6, 8497 (2015)
Erfle, H. et al. Work flow for multiplexing siRNA assays by solid-phase reverse transfection in multiwell plates. J. Biomol. Screen. 13, 575–580 (2008)
Held, M. et al. CellCognition: time-resolved phenotype annotation in high-throughput live cell imaging. Nature Methods 7, 747–754 (2010)
Acknowledgements
We thank the IMBA/IMP BioOptics microscopy facility and Molecular Biology Service, J. Meissner, and M. J. Hossain for technical support, H. Liu and S. Tietscher for generation of plasmids, C. Haering, M. Samwer, W. H. Gerlich, and O. Wueseke for comments on the manuscript, Life Science Editors for editing assistance, and U. Kutay for LAP2β-GFP/H2B–mRFP-expressing cells. D.W.G., A.A.H. and J.E. have received funding from the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreement no. 241548 (MitoSys), and A.Z.P., D.W.G. and J.E. under grant agreement no. 258068 (Systems Microscopy). D.G. has received funding from an ERC Starting Grant under agreement no. 281198 (DIVIMAGE), and from the Austrian Science Fund (FWF) project no. SFB F34-06 (Chromosome Dynamics). S.C. has received funding from a Human Frontier Science Program Long-Term Postdoctoral Fellowship and the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreement no. 330114 (IEF). T.M.R. was supported by Deutsche Forschungsgemeinschaft (DFG): SPP1384 ‘Mechanisms of Genome Haploidization’ MU 1423/3-2 and grant MU 1423/8-1.
Author information
Authors and Affiliations
Contributions
D.W.G. and S.C. conceived the project and designed experiments. B.N. and J.E. generated siRNA library transfection plates. I.P. and A.A.H. generated Ki-67–EGFP BAC cell pools. A.Z.P. and J.E. performed FCS measurements. D.W.G. and T.M.-R. performed electron microscopy experiments. S.C. performed all other experiments and C.B. assisted with the RNAi screen, chromosome spreads, cell line generation and cloning. D.W.G., S.C. and A.A.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks T. Mitchison and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Generation of a Ki-67 siRNA no. 2 resistant HeLa cell line by homozygous mutation of the endogenous Ki-67 genomic loci.
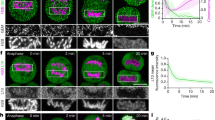
a, Quantification of chromosome area as in Fig. 1b, but displayed for each individual siRNA, showed that the three siRNAs causing strongest chromosome clustering all target Ki-67. Each data point corresponds to the median chromosome area of all live mitotic cells in a specific siRNA condition. Median, quartiles and 1.5 × interquartile range of controls (siControls and untransfected) and the siRNA library are indicated. The top four siRNAs causing an increased chromosome area all target proteins involved in cytokinesis. Hence the chromosome area increase is likely to be a consequence of polyploidization by cytokinesis failure in preceding divisions. b, Chromosome areas of all individual live mitotic cells from the original RNAi screening data. Each data point corresponds to the chromosome area in a single cell. This reveals that the clustering phenotype is very penetrant within the cell population. c, CRISPR/Cas9 nickase strategy to mutate the siKi-67 no. 2 target site without changing the amino acid coding sequence. Red triangles indicate DNA strand nicking sites. PAM, protospacer adjacent motif. sgRNA, single guide RNA. d, Schematic of genotyping strategy. A newly generated HindIII (or BglI) restriction site generated by CRISPR/Cas9 nickase as depicted in c was used to detect correctly mutated alleles. e, HindIII and BglI restriction fragments were detected by gel electrophoresis following the assay depicted in d, showing successful recombination of all three Ki-67 alleles present in HeLa cells. f, DNA sequencing chromatogram of the siKi-67 no. 2 target site of a wild-type and the CRISPR/Cas9-mutated cell line, respectively. Asterisks indicate mutated nucleotides. g, Western blot performed on whole cell lysates of wild-type or siKi-67 no. 2 resistant cell lines 48 h after the indicated siRNA transfections demonstrated that all siRNAs used in this study (siKi-67 nos 1–4) efficiently depleted Ki-67 in wild-type cells, and that the CRISPR/Cas9 mutated cell line was fully resistant against siKi-67 no. 2 but still sensitive to siKi-67 no. 1. The two bands labelled by anti Ki-67 antibody correspond to the two Ki-67 isoforms with predicted molecular masses of 320 and 359 kDa.
Extended Data Figure 2 Ki-67 is not required for initial chromosome individualization and condensation but for maintenance of chromosome separation.
a, Quantification of phenotype penetrance in Fig. 2a. Live metaphase cells recorded by an automated imaging pipeline were classified as coherent or normal by supervised machine learning (3 independent experiments with total sample numbers of n = 111 (no siRNA), n = 88 (siControl), n = 112 (siKi-67 no. 1), n = 131 (siKi-67 no. 2)). b, Western blot analysis of HeLa wild-type cells, performed at indicated time points after siKi-67 no. 1 transfection, showed efficient depletion of Ki-67 at 48–72 h post-siRNA transfection. This time window was hence used for all further experiments. For uncropped gels, see Supplementary Fig. 1. c, Live hTERT-RPE1 cells stained with Hoechst were imaged 48 h after siRNA transfection. Control cells (n = 21) had spatially separate chromosomes, whereas Ki-67 siRNA-transfected cells (n = 17) had metaphase plates that appeared as a contiguous mass of chromatin. d, Mouse embryonic stem cells were fixed 48 h after siRNA transfection and stained using anti-Ki-67 antibody and Hoechst. Metaphase plates that lacked Ki-67 signal had chromosomes merged into an unstructured mass of chromatin (lower panel), whereas all cells with residual levels of Ki-67, owing to incomplete RNAi-mediated protein depletion, had normal metaphase plate morphologies. Representative examples for n = 20 cells. e, Electron micrographs of HeLa cells transfected with siRNAs as indicated demonstrated that chromosomes appeared as a single contiguous mass. Representative images for n = 10/10 control and 9/11 for Ki-67 RNAi cells. Closed arrowheads mark dark chromatin areas; open arrowheads mark lighter grainy layer of chromosome periphery. f, Quantification of prophase chromosome condensation using pixel intensity standard deviation of the H2B–mCherry fluorescence as a measure for homogeneity of chromatin. Curves indicate mean ± s.d. of 16 cells per condition. g, Representative example cells of the chromosome condensation assay in f. h, i, Ki-67 localization on chromosomes in live prophase HeLa cells expressing H2B–mCherry/Ki-67–EGFP (h) (NEBD, t = 0 min), was quantified (excluding nucleoli) during mitotic entry (i). A significant change (P < 0.01 by Student’s t-test) in chromosome condensation (marked by red circle; compared to the initial 4 frames) occurs 11 min before a significant increase in Ki-67 localization (green circle). Curves indicate mean ± s.d. of 13 cells. Scale bars, 5 μm (e), all others 10 μm.
Extended Data Figure 3 Internal chromosome organization is not affected by Ki-67 depletion.
a, b, Hoechst-stained chromosomes of control or Ki-67-depleted HeLa cells were segmented by thresholding, subsequently a convex hull was fitted around segmented chromosomes (red line) (a) and the segmented chromatin area and area of a convex hull fit were quantified (b). The calculated chromatin area of Ki-67 depleted cells was similar to control depleted cells, which demonstrated that Ki-67 depletion has no detectable effect on chromosome-internal compaction. Ki-67 depleted cells lacked inter-chromosomal space as the convex hull area was almost identical with the chromatin area (n = 16 for each siRNA, bars indicate mean ± s.d., ****P < 0.0001 by paired Wilcoxon matched-pairs signed rank test). c, d, Live HeLa cell expressing H2B–mCherry/EGFP–Kleisin-γ were imaged 72 h after siKi-67 no. 1 or control siRNA transfection (c) and mean ± s.d. of sister chromosome axis distance were quantified (d) (n = 508 from 3 experiments (control RNAi), n = 380 from 4 experiments (Ki-67 RNAi), n = 398 from 3 experiments (no RNAi)). e, f, Live HeLa cell stably expressing H2B–mCherry/CENP-A–EGFP were imaged ~60 h after siKi-67 no. 1 or control siRNA transfection (e) and mean ± s.d. of interkinetochore distance were quantified (f) (n = 875 from 3 experiments (control RNAi), n = 1,489 from 5 experiments (Ki-67 RNAi), n = 818 from 3 experiments (no RNAi)). g, Centromere elasticity assay. Metaphase HeLa cells stably expressing H2B–mCherry/CENP-A–EGFP were imaged with 10 s time-lapse before and after nocodazole addition. The interkinetochore distance measurement over time in 12 control cells (control siRNA, light grey; untransfected cells, dark grey) and 10 cells transfected with siKi-67 no. 1 (grey lines) demonstrated that kinetochores were under tension in Ki-67 depleted cells. The red line indicates the mean. h, Chromosome spreads of untransfected or Ki-67 depleted HeLa cells appeared similar. Representative examples of 35 chromosome spreads from 2 independent experiments are depicted. i, Nuclei from control or Ki-67 depleted HeLa cells were treated with titrated amounts of MNase and DNA was subjected to 1.5% agarose electrophoresis. No detectable difference was seen in two independent experiments. Scale bars, 10 μm (a, h), 5 μm (c, e).
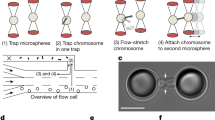
Extended Data Figure 4 Chromosome clustering and motility decrease are not caused by spatial confinement.
a, b, Representative example of kinetochore tracking performed in interphase (quantified in Fig. 2e). Live interphase HeLa cells stably expressing H2B–mCherry/CENP-A–EGFP were imaged in presence of nocodazole (a) and kinetochores were tracked in time-lapse videos (b). c, Representative MSD analyses of kinetochore tracks (CENP-A–EGFP) of mitotic HeLa cells (from Fig. 2c, d) and of the interphase cell in a. d, Western blot analysis of wild-type HeLa cells, performed at indicated time points after siKi-67 no. 1 or no. 2 transfection confirmed that both siRNAs deplete Ki-67 protein with similar efficiency. e, f, Interphase cells expressing H2B–mCherry 48 h after indicated siRNA transfections (e), were quantified with regard to nuclear size by automated segmentation of H2B–mCherry (f) (scale bars indicate mean ± s.d., ****P < 0.0001 by Kolmogorov–Smirnov test, data from 25 random positions: n = 303 (siControl), n = 303 (siKi-67 no. 1), n = 246 (siKi-67 no. 2)). Only siKi-67 no. 1 caused a significant decrease in nuclear size, while siKi-67 no. 2, which was used for kinetochore tracking in Fig. 2c–e, had no effect on nuclear size. As Ki-67 knockout cells also had a normal nuclear size (Extended Data Fig. 6f, g), we conclude that the effect of siKi-67 no. 1 on nuclear size is likely due to off-target protein depletion. g, Quantification of mitotic cell size of control or Ki-67 depleted cells demonstrated that similar to f, only siKi-67 no. 1 led to a significant decrease in cell size consistent with an off-target effect (error bars indicate mean ± s.d., ****P < 0.0001 by Mann–Whitney test, total sample numbers: n = 74 (siControl), n = 100 (siKi-67 no. 1), n = 95 (siKi-67 no. 2)). As kinetochore tracking was based on siKi-67 no. 2, the reduced motility of mitotic chromosomes cannot be attributed to cell size changes. h, Live HeLa cells stably expressing H2B–mRFP/LAP2β–EGFP were imaged 48 h after siRNA transfection as they entered mitosis in the presence of nocodazole (n = 15 per condition). Scale bars, 2 μm (a, b), 10 μm (e, h).
Extended Data Figure 5 Ki-67 depletion causes a prolonged mitosis and impairs access of spindle microtubules to chromosomes.
a, Mitotic progression determined by live-cell microscopy of HeLa cells expressing H2B–Aurora B FRET biosensor, after siRNA transfection as indicated, 3 independent experiments with total sample numbers of n = 145 (siControl), n = 97 (siKi-67 no. 2), n = 134 (siKi-67 no. 3), n = 164 (siKi-67 no. 4). b, c, Live HeLa cells stably expressing EB3–EGFP were imaged 2 min after release from a 2 h nocodazole treatment, chromosome areas were identified (yellow lines) (b) and EB3–EGFP mean fluorescence was measured in chromosome areas (c) (error bars indicate mean ± s.e.m. from 50 cells per condition, **P < 0.01 by Mann–Whitney test). Scale bar, 10 μm.
Extended Data Figure 6 Generation of a Ki-67 knockout cell line.
a, CRISPR/Cas9 was used to generate a HeLa cell line with indicated deletions on exon 5 of the Ki-67 allele. b, DNA sequencing chromatogram confirmed that no further alleles are present. c, Metaphase plates of live wild-type HeLa and Ki-67 knockout cells stained with Hoechst (n = 30 per cell line). d, Western blot performed on whole cell lysates of wild-type or Ki-67 knockout cells. The two high molecular weight bands labelled by anti-Ki-67 antibody in wild-type HeLa that corresponded to the two Ki-67 isoforms were undetectable for Ki-67 knockout cells. Actin was used as a loading control. e, Representative time-lapse image series of a Ki-67 knockout cell proceeding from prophase to prometaphase in the presence of nocodazole (n = 12, see Supplementary Video 3). Chromosomes are labelled with SiR-Hoechst and two regions were selected to exemplify coalescence of chromosomes upon their close approach. Arrows mark regions just before their coalescence. f, Automated segmentation of SiR-Hoechst-labelled interphase nuclei of wild-type and Ki-67 knockout cells confirmed a normal nuclear size of Ki-67 knockout cells (mean ± s.d. of 200 wild-type and 270 Ki-67 knockout cells). g, Representative example images of interphase wild-type or Ki-67 knockout cells stained with SiR-Hoechst of the quantification in f. h, The sensitivity of Ki-67 knockout cells to low dose nocodazole, caffeine or a topoisomerase II inhibitor (ICRF-193) was compared to wild-type by a colony formation assay. Representative images from two to three independent experiments are shown. Scale bar, 5 μm (c), 10 μm (e, g).
Extended Data Figure 7 Ki-67 has little secondary structure, is highly positively charged, and its absence can be partly compensated by overexpression of core histones.
a, Folding and charge prediction of full length Ki-67 based on FoldIndex and EMBOSS webtools using a sliding window of 100. Unfolded regions are depicted in green, folded regions in orange. Positive charge is marked in blue, negative charge in red. b, Quantification of overexpressed histone levels in individual cells related to the mitotic chromosome morphology phenotype, classified by visual inspection. Note that the mean fluorescence values are not comparable to Fig. 3c as different imaging settings had to be used. Cells are from 4–5 independent experiments. c, Radial localization of overexpressed H2B–mNeonGreen in live Ki-67 knockout cells (n = 20). Normalized fluorescence intensity along line profiles across a chromosome arm of live Ki-67 knockout cells transiently transfected with H2B–mNeonGreen (upper panel) or Ki-67–mNeonGreen (lower panel) indicate that overexpressed H2B binds to the surface as well as internal region within chromosomes. d, e, Stable association of H2B–mNeonGreen with mitotic chromosomes. d, Half of the mitotic chromosomes in Ki-67 knockout cells highly overexpressing H2B–mNeonGreen were photobleached and the recovery of fluorescence was followed by time-lapse recording in an image region (yellow box). Representative example of the quantification in e. e, Curves indicate mean ± s.d. of 20 photobleached and 19 unbleached control cells. f, Quantification of mitotic chromosome area relative to total cell area for cells shown in b and Fig. 3c. Boxes indicate median, quartiles and 1.5 × interquartile range (n = 30 for Ki-67, n = 64 for H2B). g, Live Ki-67 knockout cell transiently transfected with a H2B–mNeonGreen and stained with SiR-Hoechst. Although 25 of 64 rescued cells displayed fully separated chromosomes (Fig. 3e), a large fraction of cells (39 of 64 rescued cells) showed detectable chromosome individualization at a lower extent compared to wild-type cells. Representative single z-section of the latter is shown. h, Live Ki-67 knockout cells transiently transfected with plasmids for expression of the indicated histone fused to mNeonGreen and stained with SiR-Hoechst. Representative single z-sections of 12–18 cells from 2–3 independent experiments are depicted. Although chromosome individualization was restored, chromosomes were not separated to the same extent as in wild-type cells. Scale bars, 10 μm.
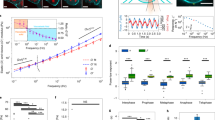
Extended Data Figure 8 Fluorescence correlation spectroscopy of endogenous Ki-67 tagged with EGFP.
a, Western blot performed on whole cell lysates of a HeLa cell line overexpressing GFP-tagged Ki-67 from a bacterial artificial chromosome (BAC), and of two different clones in which all endogenous Ki-67 alleles were N-terminally tagged with EGFP (C1, C2). The two lower bands labelled by anti-Ki-67 antibody in the BAC cell line (black arrows) correspond to the two wild-type Ki-67 isoforms. The EGFP–Ki-67 versions appear upshifted (green arrows) and the band of the small EGFP-tagged isoform overlaps with the band of the wild-type large isoform. b–h, FCS measurement from 3 independent experiments with 111 cells (C1) and 156 cells (C2). b, Imaging and analysis pipeline for FCS-calibrated imaging. Metaphase cells were identified based on Hoechst staining and imaged in 3D (only the central slice is shown). In the cytoplasm of the central slice an FCS measurement was performed. From the photon counts (right upper panel) the autocorrelation function (ACF) was computed (right lower panel). From the fit of the ACF to equation S1 in the Supplementary Information, the number of particles N in the focal volume was obtained. The concentration at the FCS point was computed by dividing N by the effective focal volume Veff and the Avogadro’s constant NA. For details see Supplementary Methods. c, A calibration curve was obtained by plotting the concentration computed from FCS against the fluorescence intensity in a 5 × 5 pixel region at the FCS measurement point (equation S2 in the Supplementary Information, dashed line). d, Fluorescence microscopy image of live HeLa cell with endogenous Ki-67, labelled by EFGP, scaled to absolute Ki-67 concentration as determined by FCS. e, Example segmentation of chromosomes and the chromosome surface used for quantification in f–h. Segmentation was performed in 3D, but for simplification only a single z-section is shown. A chromatin mask was obtained by segmentation of the Hoechst signal. To determine Ki-67 concentration on chromosomes, the mask was dilated to include Ki-67 signal at the outer chromosome surface (expanded chromatin mask). To calculate Ki-67 molecules on the outer chromosome surface, a rim around the chromatin mask was used (outer rim mask). See Supplementary Methods for details. f–h, Quantification of FCS measurements. Boxes indicate median, quartiles and 1.5 × interquartile range. f, Mean Ki-67 concentration in the cytoplasm and on 3D-segmented chromosomes using the expanded chromatin mask depicted in e. g, Total number of Ki-67 molecules within the whole DNA volume using the expanded chromatin mask depicted in e. h, Density of Ki-67 molecules on the chromosome surface using the outer rim mask depicted in e. Scale bars, 10 μm.
Extended Data Figure 9 Dual-colour labelling of the protein termini of Ki-67 indicates an extended conformation oriented perpendicular to the mitotic chromosome surface.
a, Schematic of Ki-67 labelled at the N terminus (red) and at the chromatin-binding C terminus (green). At high densities, Ki-67 might acquire extended brush-like conformations, as known for polymeric surfactants. b, Multispectral fluorescent beads with 500 nm diameter were imaged as reference data to determine dual-colour localization accuracy. The chromatic register shift in the green and red channel was corrected by image registration. c, Line profiles (white line) yielded fluorescence profiles, for green and red channels. A sum of two Gaussian functions was fitted (solid line) for each channel and peak-to-peak distances of the corresponding single Gaussian functions (dotted lines) were measured for green (d(G)) and red (d(R)) channels. d, The accuracy of the localization method as illustrated in b, c was determined based on the absolute difference between green and red peak-to-peak distances divided by 2. Mean (black line) and standard deviation (whiskers) are indicated. e–h, Representative example cells of the quantification in Fig. 4c. e, f, Live wild-type HeLa cells expressing EGFP–mCherry–Ki-67. Representative example of a sister chromatid pair oriented perpendicular to the imaging plane (e). The line profile (white line) yielded a fluorescence profile across one sister chromatid (f). A sum of two Gaussian functions was fitted (solid line) for each channel and peak-to-peak distances of the corresponding single Gaussian functions (dotted lines) were measured for green (d(G)) and red (d(R)), respectively. g, h, Live wild-type HeLa cell expressing EGFP–Ki-67–mCherry. Representative example of a sister chromatid pair oriented perpendicular to the imaging plane (g). The line profile (white line) yielded a fluorescence profile across one sister chromatid (h). A sum of two Gaussian functions was fitted (solid line) for each channel and peak-to-peak distances of the corresponding single Gaussian functions (dotted lines) were measured for green (d(G)) and red (d(R)), respectively. i, Model illustrating that an increased grafting density of Ki-67 at the chromosome surface might gradually increase the polymer brush height due to repulsive forces between the polymers. Scale bars, 1 μm.
Supplementary information
Supplementary Information
This file contains Supplementary Figure 1 (uncropped Western blots), Supplementary Methods, Supplementary Tables 2 - 4 and Supplementary References. (PDF 644 kb)
Supplementary Table 1
SiRNAs used in the screen for mitotic chromosome surface adhesion regulators (XLSX 156 kb)
Chromosome motility in Ki-67 and control depleted mitotic cells in the absence of the spindle
Mitotic monoclonal fluorescent HeLa cell line expressing H2B-mCherry/CENPA-EGFP was imaged in presence of nocodazole 70 h after indicated siRNA transfection. Bar, 10 µm. (MOV 2697 kb)
Nuclear envelope removal in Ki-67 and control depleted cells
Monoclonal fluorescent HeLa cell line expressing H2B-mRFP/LAP2β-EGFP was imaged in the presence of nocodazole 48 h after indicated siRNA transfection. Bar, 10 µm. (MOV 4070 kb)
Adhesion of chromosomes following nuclear envelope break-down in Ki-67 knockout cells
Ki-67 knock-out HeLa cell line labeled with SiR-Hoechst was imaged in presence of nocodazole starting at prophase. Bar, 10 µm. (MOV 7819 kb)
Rights and permissions
About this article
Cite this article
Cuylen, S., Blaukopf, C., Politi, A. et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 535, 308–312 (2016). https://doi.org/10.1038/nature18610
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature18610
This article is cited by
-
The evaluation of tumorigenicity and characterization of colonies in a soft agar colony formation assay using polymerase chain reaction
Scientific Reports (2023)
-
Nucleolar URB1 ensures 3′ ETS rRNA removal to prevent exosome surveillance
Nature (2023)
-
The crucial role of elasticity in regulating liquid–liquid phase separation in cells
Biomechanics and Modeling in Mechanobiology (2023)
-
Nuclear architecture and the structural basis of mitotic memory
Chromosome Research (2023)
-
Comparing the mesenchymal stem cells proliferation rate with different labeling assessments
The Nucleus (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.