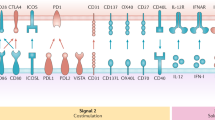
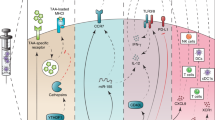
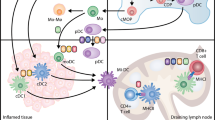
Abstract
Dendritic cells (DCs) orchestrate a repertoire of immune responses that bring about resistance to infection and silencing or tolerance to self. In the settings of infection and cancer, microbes and tumours can exploit DCs to evade immunity, but DCs also can generate resistance, a capacity that is readily enhanced with DC-targeted vaccines. During allergy, autoimmunity and transplant rejection, DCs instigate unwanted responses that cause disease, but, again, DCs can be harnessed to silence these conditions with novel therapies. Here we present some medical implications of DC biology that account for illness and provide opportunities for prevention and therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 (1973)
Banchereau, J. & Steinman, R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998)
Janeway, C. A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002)
Guermonprez, P., Valladeau, J., Zitvogel, L., Thery, C. & Amigorena, S. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20, 621–667 (2002)
Trombetta, E. S. & Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23, 975–1028 (2005)
Cyster, J. G. Chemokines and the homing of dendritic cells to the T cell areas of lymphoid organs. J. Exp. Med. 189, 447–450 (1999)
Itano, A. A. & Jenkins, M. K. Antigen presentation to naive CD4 T cells in the lymph node. Nature Immunol. 4, 733–739 (2003)
Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature Rev. Immunol. 5, 617–628 (2005)
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–780 (2001)
Probst, H. C., McCoy, K., Okazaki, T., Honjo, T. & van den Broek, M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nature Immunol. 6, 280–286 (2005)
Luo, X. et al. Dendritic cells with TGF-β1 differentiate naive CD4+CD25- T cells into islet-protective Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA 104, 2821–2826 (2007)
Pulendran, B. et al. Distinct dendritic cell subsets differentially regulate the class of immune responses in vivo. Proc. Natl Acad. Sci. USA 96, 1036–1041 (1999)
Maldonado-Lopez, R. et al. CD8α+ and CD8α- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189, 587–592 (1999)
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature Immunol. 6, 769–776 (2005)
Seder, R. A., Paul, W. E., Davis, M. M., Fazekas de St & Groth, B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J. Exp. Med. 176, 1091–1098 (1992)
Leibundgut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature Immunol. 8, 630–638 (2007)
Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192, 1213–1222 (2000)
Badovinac, V. P., Messingham, K. A., Jabbari, A., Haring, J. S. & Harty, J. T. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nature Med. 11, 748–756 (2005)
Trumpfheller, C. et al. Intensified and protective CD4+ T cell immunity at a mucosal surface after a single dose of anti-dendritic cell HIV gag fusion antibody vaccine. J. Exp. Med. 203, 607–617 (2006)
Huang, F.-P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–442 (2000)
Niess, J. H. et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307, 254–258 (2005)
Lindquist, R. L. et al. Visualizing dendritic cell networks in vivo. Nature Immunol. 5, 1243–1250 (2004)
Reis e Sousa, C. Dendritic cells in a mature age. Nature Rev. Immunol. 6, 476–483 (2006)
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007)
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nature Rev. Immunol. 7, 19–30 (2007)
Dalod, M. et al. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo.. J. Exp. Med. 195, 517–528 (2002)
Probst, H. C. & van den Broek, M. Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J. Immunol. 174, 3920–3924 (2005)
Figdor, C. G., van Kooyk, Y. & Adema, G. J. C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol. 2, 77–84 (2002)
Gautier, G. et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201, 1435–1446 (2005)
Fritz, J. H. et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity 26, 445–459 (2007)
Coutanceau, E. et al. Selective suppression of dendritic cell functions by Mycobacterium ulcerans toxin mycolactone. J. Exp. Med. 204, 1395–1403 (2007)
Querec, T. et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203, 413–424 (2006)
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 (1998)
Khader, S. A. et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 203, 1805–1815 (2006)
Geijtenbeek, T. B. H. et al. DC-SIGN, a dendritic cell specific HIV-1 binding protein that enhances trans-infection of T cells. Cell 100, 587–597 (2000)
Colonna, M., Krug, A. & Cella, M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14, 373–379 (2002)
Boscardin, S. B. et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J. Exp. Med. 203, 599–606 (2006)
Soares, H. et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo.. J. Exp. Med. 204, 1095–1106 (2007)
Bozzacco, L. et al. DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl Acad. Sci. USA 104, 1289–1294 (2007)
Delneste, Y. et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17, 353–362 (2002)
Gabrilovich, D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature Rev. Immunol. 4, 941–952 (2004)
Kortylewski, M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Med. 11, 1314–1321 (2005)
Ghiringhelli, F. et al. Tumor cells convert immature myeloid dendritic cells into TGF-β-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 202, 919–929 (2005)
Aspord, C. et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J. Exp. Med. 204, 1037–1047 (2007)
Vicari, A. P., Caux, C. & Trinchieri, G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin. Cancer Biol. 12, 33–42 (2002)
Dhodapkar, K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. Anti-tumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195, 125–133 (2002)
Kalergis, A. M. & Ravetch, J. V. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J. Exp. Med. 195, 1653–1659 (2002)
Blattman, J. N. & Greenberg, P. D. Cancer immunotherapy: a treatment for the masses. Science 305, 200–205 (2004)
Shankaran, V. et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001)
Figdor, C. G., De Vries, I. J., Lesterhuis, W. J. & Melief, C. J. Dendritic cell immunotherapy: mapping the way. Nature Med. 10, 475–480 (2004)
Banchereau, J. & Palucka, A. K. Dendritic cells as therapeutic vaccines against cancer. Nature Rev. Immunol. 5, 296–306 (2005)
Schuler-Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature, cryopreserved, peptide-loaded monocyte-derived dendritic cells. J. Exp. Med. 195, 1279–1288 (2002)
Paczesny, S. et al. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J. Exp. Med. 199, 1503–1511 (2004)
Fernandez, N. C. et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nature Med. 5, 405–411 (1999)
Lucas, M., Schachterle, W., Oberle, K., Aichele, P. & Diefenbach, A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26, 503–517 (2007)
Stary, G. et al. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 204, 1441–1451 (2007)
Palucka, A. K. et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J. Immunother. 29, 545–557 (2006)
O'Rourke, M. G. et al. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol. Immunother. 52, 387–395 (2003)
De Vries, I. J. et al. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 63, 12–17 (2003)
Banchereau, J. et al. Immune and clinical responses in patients with metastatic melanoma to CD34+ progenitor-derived dendritic cell vaccine. Cancer Res. 61, 6451–6458 (2001)
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature Med. 13, 54–61 (2007)
Gilboa, E. & Vieweg, J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol. Rev. 199, 251–263 (2004)
Soiffer, R. et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 95, 13141–13146 (1998)
Laheru, D. & Jaffee, E. M. Immunotherapy for pancreatic cancer—science driving clinical progress. Nature Rev. Cancer 5, 459–467 (2005)
Fujii, S., Liu, K., Smith, C., Bonito, A. J. & Steinman, R. M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199, 1607–1618 (2004)
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006)
Lowes, M. A. et al. Increase in TNFα and inducible nitric oxide synthase-expressing dendritic cells in psoriasis and reduction with efalizumab (anti-CD11a). Proc. Natl Acad. Sci. USA 102, 19057–19062 (2005)
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003)
Siegal, F. P. et al. The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 (1999)
Asselin-Paturel, C. & Trinchieri, G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J. Exp. Med. 202, 461–465 (2005)
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunol. 8, 487–496 (2007)
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001)
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005)
Hue, S. et al. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 (2006)
Tarbell, K. V. et al. Dendritic cell-expanded, islet-specific, CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204, 191–201 (2007)
Lambrecht, B. N. & Hammad, H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nature Rev. Immunol. 3, 994–1003 (2003)
Soumelis, V. et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nature Immunol. 3, 673–680 (2002)
Traidl-Hoffmann, C. et al. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J. Exp. Med. 201, 627–636 (2005)
Hammad, H. et al. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 204, 357–367 (2007)
Inaba, K. et al. Efficient presentation of phagocytosed cellular fragments on the MHC class II products of dendritic cells. J. Exp. Med. 188, 2163–2173 (1998)
Ochando, J. C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature Immunol. 7, 652–662 (2006)
Hackstein, H. & Thomson, A. W. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nature Rev. Immunol. 4, 24–34 (2004)
Shlomchik, W. D. et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 285, 412–415 (1999)
Merad, M. et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nature Med. 10, 510–517 (2004)
Merad, M. et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nature Immunol. 3, 1135–1141 (2002)
Rescigno, M. et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature Immunol. 2, 361–367 (2001)
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007)
Bousso, P. & Robey, E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nature Immunol. 4, 579–585 (2003)
Shakhar, G. et al. Stable T cell–dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nature Immunol. 6, 707–714 (2005)
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogation priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002)
Heath, W. R. et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199, 9–26 (2004)
Pulendran, B. Variegation of the immune response with dendritic cells and pathogen recognition receptors. J. Immunol. 174, 2457–2465 (2005)
Stranges, P. B. et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26, 629–641 (2007)
Ohteki, T. et al. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J. Exp. Med. 203, 2329–2338 (2006)
Granucci, F. et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nature Immunol. 2, 882–888 (2001)
Reis e Sousa, C. et al. In vivo microbial stimulation induces rapid CD40L- independent production of IL-12 by dendritic cells and their re-distribution to T cell areas. J. Exp. Med. 186, 1819–1829 (1997)
Sporri, R. & Reis e Sousa, C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nature Immunol. 6, 163–170 (2005)
Sallusto, F. et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 (1998)
Piqueras, B., Connolly, J., Freitas, H., Palucka, A. K. & Banchereau, J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood 107, 2613–2618 (2006)
Ito, T. et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202, 1213–1223 (2005)
Kissenpfennig, A. et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22, 643–654 (2005)
Del Rio, M. L., Rodriguez-Barbosa, J. I., Kremmer, E. & Forster, R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T Cells. J. Immunol. 178, 6861–6866 (2007)
Yoneyama, H. et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J. Exp. Med. 202, 425–435 (2005)
D'Amico, A. & Wu, L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 198, 293–303 (2003)
Karsunky, H., Merad, M., Cozzio, A., Weissman, I. L. & Manz, M. G. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198, 305–313 (2003)
Leon, B., Lopez-Bravo, M. & Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007)
Liu, K. et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nature Immunol. 8, 578–583 (2007)
Varol, C. et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204, 171–180 (2007)
Acknowledgements
The authors thank C. Moberg and M. Nussenzweig for extensive comments and J. Adams for assistance with the manuscript. We are grateful to our patients and colleagues, for their many contributions, and to the NIH and several foundations for support. A version of the review with a more complete bibliography is published on the authors’ websites: http://www.biir.org and http://www.rockefeller.edu/labheads/steinman/generalReviews.php.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.M.S is on the scientific advisory board of Celldex and Argos Therapeutics, which both design dendritic-cell-based vaccines; J.B. has stock options of Argos Therapeutics and ODC Therapy, which both focus on dendritic cells.
Rights and permissions
About this article
Cite this article
Steinman, R., Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007). https://doi.org/10.1038/nature06175
Issue Date:
DOI: https://doi.org/10.1038/nature06175
This article is cited by
-
Dendritic cell therapy for neurospoagioma: Immunomodulation mediated by tumor vaccine
Cell Death Discovery (2024)
-
Nanotechnology of inhalable vaccines for enhancing mucosal immunity
Drug Delivery and Translational Research (2024)
-
“Just right” combinations of adjuvants with nanoscale carriers activate aged dendritic cells without overt inflammation
Immunity & Ageing (2023)
-
Cardiovascular complications of diabetes: role of non-coding RNAs in the crosstalk between immune and cardiovascular systems
Cardiovascular Diabetology (2023)
-
Emerging hallmark of gliomas microenvironment in evading immunity: a basic concept
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.