Abstract
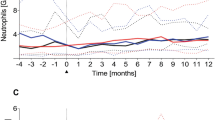
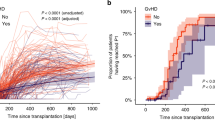
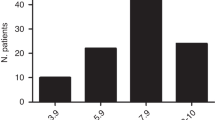
The speed of immune recovery after allo-SCT is of central importance to overcome infectious complications and relapse. To evaluate the immune reconstitution of pediatric patients concerning overall survival, we developed a three-component multivariate model and generated a reference domain of ellipsoidal shape on the basis of normal leukocyte subtype values of 100 healthy children and adolescents. The leukocyte subtypes include absolute nos. of leukocytes, CD14+ monocytes, lymphocytes, CD3+ T cells, CD3+CD4+ helper T cells, CD3+CD8+ cytotoxic T cells, CD3−CD56+ natural killer-cells and CD19+ B cells, all of which are correlated, thus, requiring the application of multivariate as opposed to multiple univariate modeling. According to their immune reconstitution, 32 pediatric patients post allo-SCT were classified into low-risk and high-risk groups on the basis of our new model. Therefore, we evaluated if the patients reached the ellipsoid of normal leukocyte sub-population values post SCT. We detected a significantly higher number of long-time survivors among the low-risk group compared with the high-risk group at days 200 (P=0.001) and 300 (P<0.0001). This is superior to our previously published univariate analysis.1 Combined with the clinical observation, a classification into risk groups based on an extended patient cohort may represent a predictor for complications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Koehl U, Bochennek K, Zimmermann SY, Lehrnbecher T, Sörensen J, Esser R et al. Immune recovery in children undergoing allogeneic stem cell transplantation: absolute CD8+CD3+ count reconstitution is associated with survival. Bone Marrow Transplant 2007; 39: 269–278.
Passweg J, Baldomero H, Leibundgut K, Schanz U, Gratwohl A . Haematopoietic stem cell transplantation in Switzerland. Swiss Med Wkly 2006; 136: 50–58.
Klingebiel T, Handgretinger R, Lang P, Bader P, Niethammer D . Haploidentical transplantation for acute lymphoblastic leukemia in childhood. Blood Rev 2004; 18: 181–192.
Devine SM, Adkins DR, Khoury H, Brown R, Vij R . Recent advances in allogeneic hematopoietic stem-cell transplantation. J Lab Clin Med 2003; 141: 7–32.
Schwinger W, Weber-Mzell D, Zois B, Rojacher T, Benesch M, Lackner H et al. Immune reconstitution after purified autologous and allogeneic blood stem cell transplantation compared with unmanipulated bone marrow transplantation in children. Brit J Haematol 2006; 135: 76–84.
Kim D, Kim J, Sohn S, Sung W, Suh J, Lee K et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Brit J Haematol 2004; 125: 217–224.
Olkinuora H, Talvensaari K, Kaartinen T, Siitonen S, Saarinen-Pihkala U, Partanen J et al. T cell regeneration in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 39: 149–156.
Comans-Bitter WM, de Groot R, van den Beemd R, Neijens H, Hop W, Groeneveld K et al. Immunophenotyping of blood lymphocytes in childhood: reference values for lymphocyte subpopulations. J Pediatrics 1997; 130: 388–393.
Huenecke S, Behl M, Fadler C, Zimmermann S, Bochennek K, Tramsen L et al. Age-matched lymphocyte subpopulation reference values in childhood and adolescence: application of exponential regression analysis. Eur J Haematol 2008; 80: 532–539.
Jolliffe I . Principal Component Analysis. Springer Series in Statistics, 2nd ed. Springer: Berlin, 2002.
Freedman LS . Tables of the number of patients required in clinical trials using the log-rank test. Stat Med 1: 121–129.
Koehl U, Gunkel M, Gruettner H, Sörensen J, Esser R . Positive selection of haematopoietic progenitor cells for autologous and allogeneic transplatation in pediatric patients with solid tumors and leukemia. Transplantation in Haematology and Oncology 1999, 1st ed. Springer editor: Berlin, pp 159–168.
Koehl U, Bochennek K, Esser R, Brinkmann A, Quaritsch R, Becker M et al. ISHAGE-based single-platform flowcytometric analysis for measurement of absolute viable T cells in fresh or cryopreserved products: CD34/CD133 selected or CD3/CD19 depleted stem cells, DLI and purified CD56+CD3-NK cells. Int J Hematol 2007; 87: 98–105.
Behringer D, Bertz H, Schmoor C, Berger C, Dwenger A, Finke J . Quantitative lymphocyte subset reconstitution after allogeneic hematopoietic transplantation from matched related donors with CD34+ selected PBPC grafts unselected PBPC grafts or BM grafts. Bone Marrow Transplant 1999; 24: 295–302.
Kalwak K, Gorczyñska E, Toporski J, Turkiewicz D, Slociak M, Ussowicz E et al. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Brit J Haematol 2002; 188: 74–89.
Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J . Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood 1998; 91: 3481–3486.
Fry T, Mackall C . Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant 2005; 35: S53–S57.
Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T . How and when should we monitor chimerism after allogeneic stem cell? Bone Marrow Transplant 2005; 35: 107–119.
Tramsen L, Beck O, Schuster F, Hunfeld K, Latge J, Sarfati J et al. Generation and characterization of anti-candida T cells as potential immunotherapy in patients with candida infection after allogeneic hematopoietic stem-cell transplant. J Infect Dis 2007; 196: 485–492.
Passweg J, Stern M, Koehl U, Uharek L, Tichelli A . Use of natural killer cells in hematopoetic stem cell transplantation. Bone Marrow Transplant 2005; 35: 637–643.
Gui J, Li H . Penalized Cox regression analysis in the high-dimensional and low sample size settings, with applications to microarray gene expression data. Bioinformatics 2005; 21: 3001–3008.
Xue X, Kim M, Shore RE . Cox regression analysis in presence of collinearity: an application to assessment of health risks associated with occupational radiation exposure. Lifetime Data Anal 2007; 13/3: 1380–7870.
Lehrnbecher T, Koehl U, Wittekindt B, Bochennek K, Tramsen L, Klingebiel T et al. Changes in host defence induced by malignancies and antineoplastic treatment: implication for immunotherapeutic strategies. Lancet Oncol 2008; 9: 269–278.
Roux E, Dumont-Girard F, Starobinski M, Siegrist C, Helg C, Chapuis B et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood 2000; 96: 2299–2303.
Eyrich M, Leiler C, Lang P, Schilbach K, Schumm M, Bader P et al. A prospective comparison of immune reconstitution in pediatric recipients. Bone Marrow Transplant 2003; 32: 379–390.
Fallen P, McGreavey L, Madrigal J, Potter M, Ethell M, Prentice H et al. Factors affecting reconstitution of the T cell compartment in allogeneic haematopoietic cell transplant recipients. Bone Marrow Transplant 2003; 32: 1001–1014.
Kook H, Goldman F, Giller R, Goeken N, Peters C, Comito M et al. Reconstruction of the immune system after unrelated or partially matched T-cell-depleted bone marrow transplantation in children: functional analyses of lymphocytes and correlation with immunophenotypic recovery following transplantation. Clin Diagn Lab Immunol 1997; 4: 96–103.
Niehues T, Rocha V, Filipovich A, Chan K, Porcher R, Michel G et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children—a eurocord analysis. Brit J Haematol 2001; 114: 42–48.
Storek J, Dawson M, Storer B, Stevens-Ayers T, Maloney D, Marr K et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 2001; 97: 3380–3389.
Kook H, Goldman F, Padley D, Giller R, Rumelhart S, Holida M et al. Reconstruction of the immune system after unrelated or partially matched T-cell-depleted bone marrow transplantation in children: immunophenotypic analysis and factors affecting the speed of recovery. Blood 1996; 88: 1089–1097.
Noel D, Witherspoon R, Storb A, Atkinson K, Doney K, Mickelson E et al. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood 1987; 51: 1087–1095.
Acknowledgements
This project was supported by ‘Deutsche Forschungsgemeinschaft (DFG, GK-1172)’, ‘Frankfurter Stiftung für Krebskranke Kinder’, ‘Adolf Messer Stiftung’, ‘Paul and Ursula Klein-Stiftung’ and ‘Alfred and Angelika Gutermuth-Stiftung’. We thank Andrea Brinkmann, Stephanie Erben, Carla Fadler, Rabiä el Kalaäoui, Sibylle Wehner, Frauke Röger and Tanja Gardlowski for the excellent technical support and Marco Dreier for data pre-processing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koenig, M., Huenecke, S., Salzmann-Manrique, E. et al. Multivariate analyses of immune reconstitution in children after allo-SCT: risk-estimation based on age-matched leukocyte sub-populations. Bone Marrow Transplant 45, 613–621 (2010). https://doi.org/10.1038/bmt.2009.204
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.204
Keywords
This article is cited by
-
Dendritic cell reconstitution is associated with relapse-free survival and acute GVHD severity in children after allogeneic stem cell transplantation
Bone Marrow Transplantation (2015)
-
Patterns of monocyte subpopulations and their surface expression of HLA-DR during adverse events after hematopoietic stem cell transplantation
Annals of Hematology (2015)
-
Feasibility of IL-15-activated cytokine-induced killer cell infusions after haploidentical stem cell transplantation
Bone Marrow Transplantation (2013)