Abstract
Background:
Experimental studies suggest potential anti-carcinogenic properties of vitamin D against breast cancer risk, but the epidemiological evidence to date is inconsistent.
Methods:
We searched MEDLINE and EMBASE databases along with a hand search for eligible studies to examine the association between vitamin D status (based on diet and blood 25-hydroxyvitamin D (25(OH)D)) and breast cancer risk or mortality in a meta-analysis. A random-effect model was used to calculate a pooled adjusted relative risk (RR).
Results:
A total of 30 prospective studies (nested case-control or cohort) were included for breast cancer incidence (n=24 studies; 31 867 cases) or mortality (n=6 studies; 870 deaths) among 6092 breast cancer patients. The pooled RRs of breast cancer incidence for the highest vs the lowest vitamin D intake and blood 25(OH)D levels were 0.95 (95% CI: 0.88–1.01) and 0.92 (95% CI: 0.83–1.02), respectively. Among breast cancer patients, high blood 25(OH)D levels were significantly associated with lower breast cancer mortality (pooled RR=0.58, 95% CI: 0.40–0.85) and overall mortality (pooled RR=0.61, 95% CI: 0.48–0.79). There was no evidence of heterogeneity and publication bias.
Conclusions:
Our findings suggest that high vitamin D status is weakly associated with low breast cancer risk but strongly associated with better breast cancer survival.
Similar content being viewed by others
Main
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related mortality among women worldwide, amounting to 23% and 14% of the total new cancer cases and deaths in 2008, respectively (Jemal et al, 2011). The most updated report from the World Cancer Research Fund (WCRF) indicated that lifestyle factors, including physical activity and alcohol drinking habit, may modify the risk of breast cancer (World Cancer Research Fund, 2010). Some dietary factors may increase or decrease the risk of breast cancer (Tirona et al, 2010), but most of the foods and nutrients, including vitamin D, listed in the report were classified as ‘Limited—no conclusion’.
Vitamin D is generally known for its major role in bone metabolism (Bouillon et al, 2008; Holick and Chen, 2008), but accumulating studies also suggest that vitamin D has anti-cancer benefits against several cancers, including breast, colorectal, and prostate cancers (Giovannucci, 2005). Ecological studies reported an inverse association between sunlight exposure and breast cancer risk (Anderson et al, 2011; Fuhrman et al, 2013). Moreover, experimental studies have found that 1,25-dihydroxyvitamin D (1,25(OH)2D), the biologically active form of vitamin D, can prevent breast cancer development and progression by inhibiting cell proliferation and angiogenesis (Krishnan et al, 2012; Lopes et al, 2012; Mohr et al, 2012). In addition, one large study conducted in Norway suggested that vitamin D from sunlight may improve the prognosis of breast, colon, and prostate cancers (Robsahm et al, 2004). Several epidemiological studies have been conducted to demonstrate the association between dietary vitamin D intake, blood 25-hydroxyvitamin D (25(OH)D) levels, and the risk of breast cancer (Gissel et al, 2008; Chen et al, 2010; Yin et al, 2010; Gandini et al, 2011; Mohr et al, 2011; Amir et al, 2012; Bauer et al, 2013; Chen et al, 2013; Wang et al, 2013) or mortality (Rose et al, 2013). There was, however, no comprehensive review and meta-analysis of observational studies examining the association between vitamin D status and breast cancer incidence as well as survival among breast cancer patients in a prospective manner. Thus, we systematically reviewed and performed a meta-analysis to quantitatively assess the association between vitamin D intake, blood 25(OH)D levels, and breast cancer incidence, along with the investigation of breast cancer mortality according to blood 25(OH)D levels among breast cancer patients.
Materials and Methods
Literature search and study identification
We conducted a literature search through the MEDLINE and EMBASE databases to identify eligible studies published in English up to November 2013. The following keywords were used in our searching: ‘(vitamin D, cholecalciferol, ergocalciferol, or 25-hydroxyvitamin D) combined with (breast cancer risk or incidence)’, and ‘(25-hydroxyvitamin D) combined with (breast cancer mortality, death, or survival).’ We also reviewed the references in the retrieved articles to search for additional relevant studies. Studies were included in the meta-analysis if they met the following criteria: (1) Studies that presented original data from cohort or nested case-control studies; (2) The outcome of interest was definitely defined as breast cancer incidence or mortality from breast cancer or all-cause mortality among breast cancer patients; (3) The exposure of interest was vitamin D intake or blood 25(OH)D levels; and (4) Studies that provided relative risks (RRs) and their confidence intervals (CIs).
Data extraction
Data were extracted using the meta-analysis of observational studies in epidemiology (MOOSE) guidelines independently by two investigators (YK and YJ) (Stroup et al, 2000). Discrepancies were resolved by discussion and repeated examination of the studies to reach a consensus. The following information was extracted from each study: first author’s last name, publication year, study design, country where the study was conducted, follow-up period or study period, number of cases and controls/subjects or person-time, adjustment for potential confounders, and RRs with corresponding 95% CIs for vitamin D intake or blood 25(OH)D levels. If studies provided several RRs, we extracted the RRs with the greatest degree of control for potential confounders.
Statistical analysis
Study-specific RRs were combined using the DerSimonian and Laird (1986) random-effects models, which considers both within- and between-study variations. If results were reported for both dietary and total vitamin D intake in one study, we used the results for total vitamin D intake in the main analysis. If original studies did not use the lowest category as a reference, the RR and its 95% CI were recalculated (Chlebowski et al, 2008; Goodwin et al, 2009; Jacobs et al, 2011; Amir et al, 2012; Neuhouser et al, 2012; Mohr et al, 2013; Ordonez-Mena et al, 2013; Vrieling et al, 2013). The summary measures were presented as forest plots where the size of data markers (squares) corresponds to the inverse of the variance of the natural logarithm of RR from each study, and the diamond indicates pooled RR. Statistical heterogeneity among studies was evaluated by using the Q statistic, and inconsistency was quantified by I2 statistic (Cochran, 1954; Higgins et al, 2003).
We conducted stratified analyses by geographic regions, menopausal status, and source of vitamin D intake (diet or supplements). To test for variations in risk estimates by the stratification factors, we carried out a meta-regression analysis. As a way to assess the quality of the prospective studies included in the meta-analysis, we calculated pooled RRs of studies with adjustment for potential confounders, such as BMI and physical activity. In addition, we performed sensitivity analyses in which one study at a time was eliminated and the rest were analysed to assess whether the results could have been influenced substantially by a single study.
To assess dose-response relationships among different categories of vitamin D intake and blood 25(OH)D, we used generalised least-squares trend (GLST) estimation analysis based on the method developed by Greenland and Longnecker (1992) (Berlin et al, 1993; Orsini et al, 2006); study-specific slopes from the correlated natural logarithm of the RRs across categories of vitamin D intake or blood 25(OH)D levels were estimated (Shin et al, 2002; McCullough et al, 2005; Lin et al, 2007; Robien et al, 2007; Chlebowski et al, 2008; Freedman et al, 2008; Rejnmark et al, 2009; Almquist et al, 2010; Engel et al, 2010, 2011; Edvardsen et al, 2011; Eliassen et al, 2011; Neuhouser et al, 2012; Kuhn et al, 2013; Mohr et al, 2013; Ordonez-Mena et al, 2013; Scarmo et al, 2013), and then we combined the GLST-estimated study-specific slopes with studies that reported slope estimates (Bertone-Johnson et al, 2005; McCullough et al, 2009) to derive an overall average slope. The highest, open-ended category was assumed to have the same amplitude of dietary intake or blood levels as the previous category. For a study which used units other than ng ml−1, we converted these into ng ml−1 (10 nmol l−1=4 ng ml−1) (Chlebowski et al, 2008; McCullough et al, 2009; Rejnmark et al, 2009; Almquist et al, 2010; Neuhouser et al, 2012; Kuhn et al, 2013). We did not include five studies that provided no information on cutoff or median of vitamin D intake in each intake category (Kuper et al, 2009), person-times in each intake category (John et al, 1999; Frazier et al, 2004; Abbas et al, 2013), or provided a risk estimate only for two categories of blood 25(OH)D levels (Amir et al, 2012) for dose-response meta-analysis.
We also examined a potential nonlinear dose-response relationship between vitamin D intake, blood 25(OH)D levels, and breast cancer by adding a quadratic term of vitamin D intake and blood 25(OH)D levels in the model. A P-value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the quadratic term is equal to 0.
Finally, publication bias was evaluated with the use of the Begg’s (Begg and Mazumdar, 1994) and Egger’s tests (Egger et al, 1997) and through visual inspection of a funnel plot. A two-tailed P value of<0.05 was considered statistically significant. All statistical analyses were performed with the Stata/SE version 12.0 software (Stata Corporation, College Station, TX, USA).
Results
Study characteristics
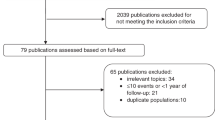
The detailed steps of our literature search are shown in Figure 1. In brief, a total of 30 prospective studies (nested case-control or cohort) were included for breast cancer incidence or mortality among breast cancer patients. The characteristics of the included studies are summarised in Tables 1, 2, 3. For breast cancer risk in relation to vitamin D intake, we included 10 prospective cohort studies, including 22 341 incident cases (John et al, 1999; Shin et al, 2002; Frazier et al, 2004; McCullough et al, 2005; Lin et al, 2007; Robien et al, 2007; Kuper et al, 2009; Edvardsen et al, 2011; Engel et al, 2011; Abbas et al, 2013; Table 1). Six studies were performed in the United States (John et al, 1999; Shin et al, 2002; Frazier et al, 2004; McCullough et al, 2005; Lin et al, 2007; Robien et al, 2007), whereas four studies were conducted in Europe (Kuper et al, 2009; Edvardsen et al, 2011; Engel et al, 2011; Abbas et al, 2013). Five out of the 10 studies presented risk estimates stratified by menopausal status of the study subjects (Shin et al, 2002; Lin et al, 2007; Robien et al, 2007; Engel et al, 2011; Abbas et al, 2013). Most of the studies provided risk estimates that were adjusted for potential confounders, including BMI (John et al, 1999; Shin et al, 2002; Frazier et al, 2004; Lin et al, 2007; Robien et al, 2007; Kuper et al, 2009; Edvardsen et al, 2011; Engel et al, 2011) and physical activity (John et al, 1999; Shin et al, 2002; Lin et al, 2007; Robien et al, 2007; Kuper et al, 2009; Engel et al, 2011; Abbas et al, 2013). One study did not adjust for BMI, but they did adjust for weight and height, which may be considered a proxy for BMI (Abbas et al, 2013).
For breast cancer risk in relation to blood 25(OH)D levels, 14 prospective studies (13 nested case-control and 1 cohort), including 9526 incident cases, were included (Bertone-Johnson et al, 2005; Chlebowski et al, 2008; Freedman et al, 2008; McCullough et al, 2009; Rejnmark et al, 2009; Almquist et al, 2010; Engel et al, 2010; Eliassen et al, 2011; Amir et al, 2012; Neuhouser et al, 2012; Kuhn et al, 2013; Mohr et al, 2013; Ordonez-Mena et al, 2013; Scarmo et al, 2013; Table 2). Eight studies were conducted in the North America (USA/Canada) (Bertone-Johnson et al, 2005; Chlebowski et al, 2008; Freedman et al, 2008; McCullough et al, 2009; Eliassen et al, 2011; Amir et al, 2012; Neuhouser et al, 2012; Mohr et al, 2013), five in Europe (Rejnmark et al, 2009; Almquist et al, 2010; Engel et al, 2010; Kuhn et al, 2013; Ordonez-Mena et al, 2013), and one study provided overall results from USA and Sweden (Scarmo et al, 2013). Ten out of the 14 studies presented risk estimates stratified by menopausal status of the study subjects (Chlebowski et al, 2008; Freedman et al, 2008; McCullough et al, 2009; Rejnmark et al, 2009; Almquist et al, 2010; Engel et al, 2010; Eliassen et al, 2011; Neuhouser et al, 2012; Ordonez-Mena et al, 2013; Scarmo et al, 2013), and the majority of studies adjusted for BMI (Bertone-Johnson et al, 2005; Chlebowski et al, 2008; Freedman et al, 2008; McCullough et al, 2009; Almquist et al, 2010; Engel et al, 2010; Eliassen et al, 2011; Amir et al, 2012; Neuhouser et al, 2012; Kuhn et al, 2013; Ordonez-Mena et al, 2013; Scarmo et al, 2013), and 6 studies were adjusted for physical activity (Chlebowski et al, 2008; Engel et al, 2010; Neuhouser et al, 2012; Kuhn et al, 2013; Ordonez-Mena et al, 2013; Scarmo et al, 2013).
In addition, we found six prospective studies that examined mortality in relation to blood 25(OH)D levels among breast cancer patients (Table 3; Goodwin et al, 2009; Jacobs et al, 2011; Hatse et al, 2012; Tretli et al, 2012; Villasenor et al, 2013; Vrieling et al, 2013). The studies included 870 overall deaths among 6092 patients and 301 deaths from breast cancer among 4556 patients. According to geographic region, three studies were performed in North America (Goodwin et al, 2009; Jacobs et al, 2011; Villasenor et al, 2013) and three in Europe (Hatse et al, 2012; Tretli et al, 2012; Vrieling et al, 2013). Three out of the six studies provided multivariable risk estimates adjusted for BMI (Jacobs et al, 2011; Hatse et al, 2012; Villasenor et al, 2013). One study did try adjustment for BMI initially, but the BMI was left out as an adjustment factor in the final model as the result was not changed materially after the adjustment (Vrieling et al, 2013).
High vs low vitamin D intake or 25(OH)D levels for breast cancer risk
The multivariable-adjusted RRs of breast cancer risk for each study and the pooled RR from all studies combined for the highest vs lowest categories of vitamin D intake or blood 25(OH)D levels are shown in Figures 2 and 3. The pooled RR of breast cancer for the highest (>500 IU day−1, mean) vs lowest categories of vitamin D intake (<148 IU day−1, mean) was 0.95 (95% CI: 0.88–1.01), with no significant heterogeneity among the studies (P=0.09, I2=38.3%) (Figure 2). No significant differences were found by geographic region (P=0.31), menopausal status (P=0.37), or source of vitamin D intake (P=0.33) (Supplementary Table). When limited to studies that had adjusted for BMI (or weight and height) or physical activity, the pooled RRs were similar (pooled RR adjusted for BMI=0.94, 95% CI: 0.88–1.02; pooled RR adjusted for PA=0.92, 95% CI: 0.85–0.99) (data not shown). The pooled RR of breast cancer for the highest (>31 ng ml−1, mean) vs lowest categories of 25(OH)D levels (<18 ng ml−1, mean) was 0.92 (95% CI: 0.83–1.02), with no significant heterogeneity among the studies (P=0.16, I2=27.3%) (Figure 3). When we excluded studies that did not adjust for BMI or physical activity, the results of meta-analyses were similar. In the subgroup analyses, we found no significant variations in pooled RRs by geographic region (P=0.73) or menopausal status (P=0.37) (Supplementary Table).
High vs low 25(OH)D levels for mortality among breast cancer patients
The multivariable-adjusted RRs of mortality for each study and the pooled RR from all studies combined for the highest vs lowest categories of blood 25(OH)D levels are shown in Figures 4 and 5. Among breast cancer patients, women with high blood 25(OH)D levels (>29.1 ng ml−1, mean) were significantly associated with lower mortality from breast cancer (n=4 studies) compared with those who had low blood 25(OH)D levels (<21 ng ml−1, mean) (pooled RR=0.58, 95% CI: 0.40–0.85; Figure 4). Similarly, for overall mortality, (n=6 studies), the pooled RR for the highest (>27.5 ng ml−1, mean) vs lowest (<20.7 ng ml−1, mean) categories of blood 25(OH)D levels was 0.61 (95% CI: 0.48–0.79; Figure 5). No significant heterogeneity among the studies was found in the meta-analyses (breast cancer mortality: P=0.25, I2=26.7%; overall mortality: P=0.17, I2=35.9%). When we excluded one study that did not adjust for BMI (Tretli et al, 2012), the pooled RR of overall mortality was 0.67 (95% CI: 0.51–0.90). For the risk of breast cancer recurrence (n=3 studies), we also found a significant inverse association (for>26.9 vs<14.7 ng ml−1, mean: pooled RR=0.61, 95% CI: 0.47–0.80) (data not shown).
Dose-response meta-analysis
For the dose-response analysis of breast cancer risk, 6 and 13 studies were included for the analysis of vitamin D intake and 25(OH)D levels, respectively. The pooled RRs of breast cancer risk for a 100-IU day−1 increment in vitamin D intake and 10 ng ml−1 increment in blood 25(OH)D levels were 0.99 (95% CI: 0.98–1.00) and 0.98(95% CI: 0.96–1.00), with no significant heterogeneity among the studies. For mortality among breast cancer patients, the pooled RRs for a 10-ng ml−1 increment in blood 25(OH) D levels were 0.88 (95% CI: 0.79-0.98) for breast cancer mortality (n=3 studies) and 0.84 (95% CI: 0.78–0.91) for overall mortality (n=3 studies), respectively. No significant heterogeneity among the studies were found (breast cancer mortality: P=0.27, I2=23.2%; overall mortality: P=0.31, I2=15.0%).
Publication bias
There was no indication of publication bias in the literature on breast cancer risk and vitamin D intake (Begg’s P=0.68, Egger’s P=0.33) or blood 25(OH)D levels (Begg’s P=0.74, Egger’s P=0.46). For mortality from breast cancer or all-cause mortality among patients, we found no evidence of publication bias either (breast cancer mortality: Begg’s P>0.99, Egger’s P=0.38; overall mortality: Begg’s P=0.85, Egger’s P=0.90).
Discussion
The current meta-analysis assessed the association between vitamin D intake/blood 25(OH)D levels and breast cancer risk or mortality based on 30 prospective studies. We found an overall nonsignificant, weak inverse association between vitamin D intake or 25(OH)D levels and breast cancer risk. Among breast cancer patients, however, high blood 25(OH)D levels were significantly associated with low breast cancer mortality. The risk of death from breast cancer decreased by 42% for high (>29.1 ng ml−1, mean) vs low 25(OH)D levels (<21 ng ml−1, mean). Overall, there was no significant heterogeneity among the studies.
Vitamin D can be obtained from foods or supplements, but endogenous production of vitamin D is an important source as well. When our skin is exposed to UV light, Vitamin D3 is synthesised via the initial conversation of 7-dyhydrocholesterol, and then within 48 h, the liver hydroxylates all vitamin D to 25(OH)D, which has a biological half-life of at least 2 months (Knight et al, 2007; Moukayed and Grant, 2013). A biologically active form of vitamin D, 1,25(OH)2D, can be produced in many tissues, including the breast as well as the kidney, by 25(OH)D-1α-hydroxylase (Zehnder et al, 2001). The active hormone has relatively a short half-life (4–6 h) and is tightly regulated by the kidneys to maintain calcium homeostasis. In addition, 1,25(OH)2D levels are often normal or even elevated in vitamin D-deficient patients as a result of secondary hyperparathyroidism (Holick, 2007). Thus, plasma 25(OH)D rather than 1,25(OH)2D is a more appropriate measure to determine vitamin D status for the human body (Holick, 2009). The association of breast cancer risk in relation to high vs low vitamin D intake from diet and supplements (pooled RR=0.95) was slightly weaker than the association with 25(OH)D levels (pooled RR=0.92) in our meta-analyses, both of which estimates were not statistically significant. As relatively small amounts of vitamin D can be obtained through a limited number of dietary sources such as fatty fish and fortified milk (Knight et al, 2007), the effect estimate for disease risk tends to be stronger in studies using blood 25(OH)D than vitamin D intake. A previous meta-analysis of vitamin D intake showed a significant inverse association between high vs low vitamin D intake and breast cancer risk (RR=0.91, 95% CI: 0.85–0.97), but the study included case-control studies that are susceptible to methodological biases (Chen et al, 2010), while our results were based on 10 prospective cohort studies. When we conducted stratified meta-analyses according to the source of vitamin D (diet or supplements), the association with supplemental vitamin D intake was slightly stronger (pooled RR=0.91, 95% CI: 0.84–1.00) than the association with dietary vitamin D intake (pooled RR=0.96; 95% CI: 0.89–1.03), which was consistent with the previous report (Chen et al, 2010). The difference in the risk estimates, however, did not vary by sources of vitamin D substantially (P for difference=0.33).
The recent meta-analysis of nine prospective studies to examine 25(OH)D levels and breast cancer risk showed a significant inverse association among postmenopausal women (RR per 5 ng ml−1=0.97, 95% CI: 0.93–1.00) but not among premenopausal women (RR per 5 ng ml−1=1.01, 95% CI: 0.98–1.04) (P=0.05 for effect modification) (Bauer et al, 2013). In our meta-analysis of 14 prospective studies for 25(OH)D levels and breast cancer risk, additional 6 prospective studies (Neuhouser et al, 2012; Amir et al, 2012; Kuhn et al, 2013; Mohr et al, 2013; Ordonez-Mena et al, 2013; Scarmo et al, 2013) were included, while 1 study conducted on pregnant women was not included (Agborsangaya et al, 2010). Our meta-analysis stratified by menopausal status tended to show slightly stronger inverse association among premenopausal women than among postmenopausal women, but the difference did not vary substantially, the tendency of which was similar for the analysis of vitamin D intake as well. The recent pooled analysis of two randomised clinical trials (Lappe et al, 2007; Avenell et al, 2012) focusing on vitamin D supplementation in breast cancer prevention did not support a role of vitamin D in reducing breast cancer risk in postmenopausal women, which might be due, in part, to dose inadequacy and insufficient study length of the trials to the detection of incident cancer cases (Sperati et al, 2013).
We also conducted a meta-analysis after restricting to studies that had adjusted for BMI or physical inactivity, which are well-known risk factors for breast cancer (Key et al, 2003; Renehan et al, 2008; Steindorf et al, 2013; Wu et al, 2013). Higher BMI generally means more body fat, and body fatness directly affects levels of serum concentrations of some hormones such as oestrogens, insulin, and insulin-like growth factors, which may facilitate breast carcinogenesis (Key et al, 2003). These hormones contribute to the development of breast cancer by stimulating the body’s inflammatory response (Howe et al, 2013). Physical activity seems to reduce the risk of breast cancer by directly decreasing the levels of serum oestrogens (McTiernan et al, 2004) and serum insulin (Borghouts and Keizer, 2000) or through the reduction of body fat. BMI and physical activity were also associated with vitamin D status. People who exercise regularly are more likely to be exposed to sunlight, which elevates blood 25(OH)D levels. Higher BMI has been found to be associated with lower concentrations of blood 25(OH)D levels (Muscogiuri et al, 2010; Vaidya et al, 2012), which is probably due to decreased bioavailability of vitamin D resulting from deposition in adipose tissue (Wortsman et al, 2000; Earthman et al, 2012). As BMI and physical activity are potential confounders for the association between vitamin D and breast cancer risk, studies that adjusted for BMI or physical activity can provide more accurate risk estimates to determine the association. The nonsignificant, weak inverse association of vitamin D intake and breast cancer risk that we found in the main analysis tended to be stronger in the secondary analyses of studies that adjusted for BMI or physical activity.
In terms of mortality from breast cancer or all-cause mortality, we found significant inverse associations between vitamin D status and cancer prognosis, which was consistent with the previous report (Rose et al, 2013). There are some potential mechanisms through which high blood levels of 25(OH)D may improve survival among breast cancer patients. Vitamin D receptors, which are activated by 1,25(OH)2D, control a variety of cellular mechanisms with respect to cancer development such as differentiation, cell proliferation, apoptosis, angiogenesis, and metastatic potential (Welsh, 2004; Mohr et al, 2012). Some experimental studies reported that vitamin D analogs suppressed tumour growth in mouse model with breast cancer (Sundaram et al, 2003; Lee et al, 2008; Ooi et al, 2010; So et al, 2011). In addition, 1,25(OH)2D inhibited breast cancer cell growth by reducing oestrogen levels (Swami et al, 2012), which is achieved through repressing the expression of genes related to oestrogen synthesis (Stoica et al, 1999; Swami et al, 2000, 2012; Krishnan et al, 2010). Another potential anti-cancer activity of vitamin D is related to prostaglandin, which stimulates tumour progression by increasing proliferation and angiogenesis (Wang and Dubois, 2004; Cordes et al, 2012). 1,25(OH)2D repressed the expression of cyclooxygenase-2, which has an important role in prostaglandin synthesis (Moreno et al, 2005; Krishnan et al, 2010). In addition, vitamin D sufficiency seems to suppress downregulation of E-cadherin, a glycoprotein that helps to keep cells in close contact and thus a well-differentiated state, which occurs in vitamin D deficiency, thereby improving breast cancer prognosis (Berx and Van Roy, 2001; Mohr et al, 2012).
Our study has some strengths. This quantitative assessment was based on prospective studies. The design of case-control studies are more prone to methodological biases such as recall bias and selection bias, which limits the strength and quality of the evidence, and our meta-analysis of prospective studies can overcome the shortcoming of the retrospective studies. The included studies in the meta-analysis of breast cancer risk measured vitamin D intake or blood 25(OH)D levels before breast cancer is diagnosed, so that the possibility that cancer status affects vitamin D status can be minimised. Furthermore, we performed a comprehensive meta-analysis of vitamin D related to breast cancer survival as well as breast cancer incidence. In the breast cancer risk analysis, both measurements of vitamin D intake (from food and/or supplements) and circulating 25(OH)D levels were used. We also reported the results of 25(OH)D levels for the risk of breast cancer mortality or overall mortality among breast cancer patients, which has been less thoroughly investigated compared with the analyses of breast cancer risk in healthy women. We found no evidence of either significant heterogeneity among the studies or any publication bias in our meta-analyses, which may suggest that our results are robust.
Despite these strengths, our study also has several limitations. First, some misclassification of vitamin D status may exist, which influences the results of individual studies and thus pooled estimates in this meta-analyses. For the measurements of vitamin D intake or blood 25(OH)D levels, most of the studies included in the meta-analyses used a single measurement at baseline, which could lead to an underestimation of risk estimates. Three studies updated the information about diet during study periods (Shin et al, 2002; McCullough et al, 2005; Robien et al, 2007), which can reduce the possibility of exposure misclassification. There is one study showing that risk estimates tend to be weaker as follow-up period increases (Grant, 2011). It is possible that our pooled estimates would be likely to be stronger if repeated measurement of vitamin D status were available in each study. There was a strong inverse association with vitamin D levels for breast cancer risk among case-control studies (Yin et al, 2010; Amir et al, 2012; Chen et al, 2013), and this may be due, in part, to less exposure misclassification as there was a relatively short time interval between blood collection and breast cancer diagnosis in the design of case-control studies. Nonetheless, we cannot rule out the possibility that lower vitamin D level for the cancer patients was influenced by breast cancer presence and stage among case-control studies where blood was collected after lower breast cancer diagnosis (Chlebowski, 2013). Second, unmeasured or residual confounding may still affect the risk estimates in each study and thus pooled estimates in the meta-analyses, although the majority of studies tried to adjust for important confounders in the multivariable models. When we conducted meta-analyses of studies that adjusted for BMI or physical activity, the inverse association with vitamin D intake tended to be stronger. Third, a relatively small number of studies were included for the analysis of mortality among breast cancer patients, which was due to limited number of data published. Nevertheless, we found a significant inverse association between vitamin D status and breast cancer mortality outcome. Fourth, the cutoffs for high and low vitamin D categories varied among the studies, which is often considered as a limitation inherent in the meta-analysis. However, we found no significant heterogeneity among the studies for high vs low vitamin D analyses, and we also conducted a dose-response meta-analysis. Results of a sensitivity analysis excluding one study at a time (e.g., including the exclusion of Almquist et al (2010) that used relatively high levels of 25(OH)D as a cutoff in the lowest category) showed that not one single study affected the pooled RR substantially. Finally, our search was restricted to studies published in English, so language bias remains a possibility.
In conclusion, findings from this meta-analysis of 30 prospective studies suggest that high vitamin D status is weakly associated with low risk of breast cancer but strongly associated with better cancer survival among breast cancer patients. As studies consistently report a high prevalence of relatively low 25(OH)D levels in breast cancer patients (Neuhouser et al, 2008; Chlebowski, 2013), we may recommend them to increase their vitamin D levels by considering taking a vitamin D supplementation to achieve optimal levels (30–50 ng ml−1 as recommended by the Institute of Medicine). For every 100 IU of vitamin D, blood 25(OH) D levels increase by 1 ng ml−1, and most experts agree that a minimum of 1000 IU day−1 vitamin D is needed to have a preferred healthy level of >30 ng ml−1 of blood 25(OH)D, which is difficult to be achieved without supplementation (Heaney, 2008; Heaney et al, 2003). As all of the studies included in the meta-analysis were observational and research evidence from clinical trials was limited, current evidence does not support use of high dose vitamin D regimens to get benefits for breast cancer survival. More large randomised clinical trials with sufficient study length and dose adequacy should be conducted to provide definitive evidence and have implications for clinical practice.
Change history
27 May 2014
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abbas S, Linseisen J, Rohrmann S, Chang-Claude J, Peeters PH, Engel P, Brustad M, Lund E, Skeie G, Olsen A, Tjonneland A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G, Kaaks R, Boeing H, Buijsse B, Adarakis G, Ouranos V, Trichopoulou A, Masala G, Krogh V, Mattiello A, Tumino R, Sacerdote C, Buckland G, Suarez MV, Sanchez MJ, Chirlaque MD, Barricarte A, Amiano P, Manjer J, Wirfalt E, Lenner P, Sund M, Bueno-de-Mesquita HB, van Duijnhoven FJ, Khaw KT, Wareham N, Key TJ, Fedirko V, Romieu I, Gallo V, Norat T, Wark PA, Riboli E (2013) Dietary intake of vitamin d and calcium and breast cancer risk in the European prospective investigation into cancer and nutrition. Nutr Cancer 65 (2): 178–187.
Agborsangaya CB, Surcel HM, Toriola AT, Pukkala E, Parkkila S, Tuohimaa P, Lukanova A, Lehtinen M (2010) Serum 25-hydroxyvitamin D at pregnancy and risk of breast cancer in a prospective study. Eur J Cancer 46 (3): 467–470.
Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J (2010) Serum levels of vitamin D, PTH and calcium and breast cancer risk—a prospective nested case-control study. Int J Cancer 127 (9): 2159–2168.
Amir E, Cecchini RS, Ganz PA, Costantino JP, Beddows S, Hood N, Goodwin PJ (2012) 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res Treat 133 (3): 1077–1088.
Anderson LN, Cotterchio M, Kirsh VA, Knight JA (2011) Ultraviolet sunlight exposure during adolescence and adulthood and breast cancer risk: a population-based case-control study among Ontario women. Am J Epidemiol 174 (3): 293–304.
Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA RECORD Trial Group (2012) Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab 97 (2): 614–622.
Bauer SR, Hankinson SE, Bertone-Johnson ER, Ding EL (2013) Plasma vitamin D levels, menopause, and risk of breast cancer: dose-response meta-analysis of prospective studies. Medicine (Baltimore) 92 (3): 123–131.
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50 (4): 1088–1101.
Berlin JA, Longnecker MP, Greenland S (1993) Meta-analysis of epidemiologic dose-response data. Epidemiology 4 (3): 218–228.
Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE (2005) Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 14 (8): 1991–1997.
Berx G, Van Roy F (2001) The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 3 (5): 289–293.
Borghouts LB, Keizer HA (2000) Exercise and insulin sensitivity: a review. Int J Sports Med 21 (1): 1–12.
Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M (2008) Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29 (6): 726–776.
Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H (2010) Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat 121 (2): 469–477.
Chen P, Li M, Gu X, Liu Y, Li X, Li C, Wang Y, Xie D, Wang F, Yu C, Li J, Chen X, Chu R, Zhu J, Ou Z, Wang H (2013) Higher blood 25(OH)D level may reduce the breast cancer risk: evidence from a Chinese population based case-control study and meta-analysis of the observational studies. PLoS One 8 (1): e49312.
Chlebowski RT (2013) Vitamin D and breast cancer incidence and outcome. Anticancer Agents Med Chem 13 (1): 98–106.
Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O'Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA Women's Health Initiative I (2008) Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 100 (22): 1581–1591.
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10: 101–129.
Cordes T, Hoellen F, Dittmer C, Salehin D, Kummel S, Friedrich M, Koster F, Becker S, Diedrich K, Thill M (2012) Correlation of prostaglandin metabolizing enzymes and serum PGE2 levels with vitamin D receptor and serum 25(OH)2D3 levels in breast and ovarian cancer. Anticancer Res 32 (1): 351–357.
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7 (3): 177–188.
Earthman CP, Beckman LM, Masodkar K, Sibley SD (2012) The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 36 (3): 387–396.
Edvardsen K, Veierod MB, Brustad M, Braaten T, Engelsen O, Lund E (2011) Vitamin D-effective solar UV radiation, dietary vitamin D and breast cancer risk. Int J Cancer 128 (6): 1425–1433.
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109): 629–634.
Eliassen AH, Spiegelman D, Hollis BW, Horst RL, Willett WC, Hankinson SE (2011) Plasma 25-hydroxyvitamin D and risk of breast cancer in the Nurses' Health Study II. Breast Cancer Res 13 (3): R50.
Engel P, Fagherazzi G, Boutten A, Dupre T, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F (2010) Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev 19 (9): 2341–2350.
Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F (2011) Joint effects of dietary vitamin D and sun exposure on breast cancer risk: results from the French E3N cohort. Cancer Epidemiol Biomarkers Prev 20 (1): 187–198.
Frazier AL, Li L, Cho E, Willett WC, Colditz GA (2004) Adolescent diet and risk of breast cancer. Cancer Causes Control 15 (1): 73–82.
Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG (2008) Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev 17 (4): 889–894.
Fuhrman BJ, Freedman DM, Bhatti P, Doody MM, Fu YP, Chang SC, Linet MS, Sigurdson AJ (2013) Sunlight, polymorphisms of vitamin D-related genes and risk of breast cancer. Anticancer Res 33 (2): 543–551.
Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P (2011) Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 128 (6): 1414–1424.
Giovannucci E (2005) The epidemiology of vitamin D and cancer incidence and mortality: a review (United States). Cancer Causes Control 16 (2): 83–95.
Gissel T, Rejnmark L, Mosekilde L, Vestergaard P (2008) Intake of vitamin D and risk of breast cancer—a meta-analysis. J Steroid Biochem Mol Biol 111 (3-5): 195–199.
Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N (2009) Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol 27 (23): 3757–3763.
Grant WB (2011) Effect of interval between serum draw and follow-up period on relative risk of cancer incidence with respect to 25-hydroxyvitamin D level: Implications for meta-analyses and setting vitamin D guidelines. Dermatoendocrinol 3 (3): 199–204.
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135 (11): 1301–1309.
Hatse S, Lambrechts D, Verstuyf A, Smeets A, Brouwers B, Vandorpe T, Brouckaert O, Peuteman G, Laenen A, Verlinden L, Kriebitzsch C, Dieudonne AS, Paridaens R, Neven P, Christiaens MR, Bouillon R, Wildiers H (2012) Vitamin D status at breast cancer diagnosis: correlation with tumor characteristics, disease outcome, and genetic determinants of vitamin D insufficiency. Carcinogenesis 33 (7): 1319–1326.
Heaney RP (2008) Vitamin D in health and disease. Clin J Am Soc Nephrol 3 (5): 1535–1541.
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77 (1): 204–210.
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327 (7414): 557–560.
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266–281.
Holick MF (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19 (2): 73–78.
Holick MF, Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87 (4): 1080S–1086S.
Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ (2013) Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res 19 (22): 6074–6083.
Jacobs ET, Thomson CA, Flatt SW, Al-Delaimy WK, Hibler EA, Jones LA, Leroy EC, Newman VA, Parker BA, Rock CL, Pierce JP (2011) Vitamin D and breast cancer recurrence in the Women's Healthy Eating and Living (WHEL) Study. Am J Clin Nutr 93 (1): 108–117.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61 (2): 69–90.
John EM, Schwartz GG, Dreon DM, Koo J (1999) Vitamin D and breast cancer risk: the NHANES I epidemiologic follow-up study, 1971-1975 to 1992. Cancer Epidemiol Biomarkers Prev 8 (5): 399–406.
Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE Jr., Falk RT, Miller R, Schatzkin A, Allen DS, Fentiman IS, Key TJ, Wang DY, Dowsett M, Thomas HV, Hankinson SE, Toniolo P, Akhmedkhanov A, Koenig K, Shore RE, Zeleniuch-Jacquotte A, Berrino F, Muti P, Micheli A, Krogh V, Sieri S, Pala V, Venturelli E, Secreto G, Barrett-Connor E, Laughlin GA, Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE, Cauley JA, Kuller LH, Cummings SR, Helzlsouer KJ, Alberg AJ, Bush TL, Comstock GW, Gordon GB, Miller SR, Longcope C Endogenous Hormones Breast Cancer Collaborative G (2003) Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 95 (16): 1218–1226.
Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R (2007) Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 16 (3): 422–429.
Krishnan AV, Swami S, Feldman D (2012) The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids 77 (11): 1107–1112.
Krishnan AV, Swami S, Peng L, Wang J, Moreno J, Feldman D (2010) Tissue-selective regulation of aromatase expression by calcitriol: implications for breast cancer therapy. Endocrinology 151 (1): 32–42.
Kuhn T, Kaaks R, Becker S, Eomois PP, Clavel-Chapelon F, Kvaskoff M, Dossus L, Tjonneland A, Olsen A, Overvad K, Chang-Claude J, Lukanova A, Buijsse B, Boeing H, Trichopoulou A, Lagiou P, Bamia C, Masala G, Krogh V, Sacerdote C, Tumino R, Mattiello A, Buckland G, Sanchez MJ, Menendez V, Chirlaque MD, Barricarte A, Bueno-de-Mesquita HB, van Duijnhoven FJ, van Gils CH, Bakker MF, Weiderpass E, Skeie G, Brustad M, Andersson A, Sund M, Wareham N, Khaw KT, Travis RC, Schmidt JA, Rinaldi S, Romieu I, Gallo V, Murphy N, Riboli E, Linseisen J (2013) Plasma 25-hydroxyvitamin D and the risk of breast cancer in the European prospective investigation into cancer and nutrition: a nested case-control study. Int J Cancer 133 (7): 1689–1700.
Kuper H, Yang L, Sandin S, Lof M, Adami HO, Weiderpass E (2009) Prospective study of solar exposure, dietary vitamin D intake, and risk of breast cancer among middle-aged women. Cancer Epidemiol Biomarkers Prev 18 (9): 2558–2561.
Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP (2007) Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85: 1586–1591.
Lee HJ, Paul S, Atalla N, Thomas PE, Lin X, Yang I, Buckley B, Lu G, Zheng X, Lou YR, Conney AH, Maehr H, Adorini L, Uskokovic M, Suh N (2008) Gemini vitamin D analogues inhibit estrogen receptor-positive and estrogen receptor-negative mammary tumorigenesis without hypercalcemic toxicity. Cancer Prev Res (Phila) 1 (6): 476–484.
Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM (2007) Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med 167 (10): 1050–1059.
Lopes N, Paredes J, Costa JL, Ylstra B, Schmitt F (2012) Vitamin D and the mammary gland: a review on its role in normal development and breast cancer. Breast Cancer Res 14 (3): 211.
McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE (2005) Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 14 (12): 2898–2904.
McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE (2009) Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res 11 (4): R64.
McTiernan A, Tworoger SS, Ulrich CM, Yasui Y, Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, Potter JD, Schwartz RS (2004) Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res 64 (8): 2923–2928.
Mohr SB, Gorham ED, Alcaraz JE, Kane CJ, Macera CA, Parsons JK, Wingard DL, Garland CF (2011) Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res 31 (9): 2939–2948.
Mohr SB, Gorham ED, Alcaraz JE, Kane CI, Macera CA, Parsons JK, Wingard DL, Garland CF (2012) Does the evidence for an inverse relationship between serum vitamin D status and breast cancer risk satisfy the Hill criteria? Dermatoendocrinol 4 (2): 152–157.
Mohr SB, Gorham ED, Alcaraz JE, Kane CI, Macera CA, Parsons JK, Wingard DL, Horst R, Garland CF (2013) Serum 25-hydroxyvitamin D and breast cancer in the military: a case-control study utilizing pre-diagnostic serum. Cancer Causes Control 24 (3): 495–504.
Moreno J, Krishnan AV, Swami S, Nonn L, Peehl DM, Feldman D (2005) Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res 65 (17): 7917–7925.
Moukayed M, Grant WB (2013) Molecular link between vitamin D and cancer prevention. Nutrients 5 (10): 3993–4021.
Muscogiuri G, Sorice GP, Prioletta A, Policola C, Della Casa S, Pontecorvi A, Giaccari A (2010) 25-Hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 18 (10): 1906–1910.
Neuhouser ML, Manson JE, Millen A, Pettinger M, Margolis K, Jacobs ET, Shikany JM, Vitolins M, Adams-Campbell L, Liu S, LeBlanc E, Johnson KC, Wactawski-Wende J (2012) The influence of health and lifestyle characteristics on the relation of serum 25-hydroxyvitamin D with risk of colorectal and breast cancer in postmenopausal women. Am J Epidemiol 175 (7): 673–684.
Neuhouser ML, Sorensen B, Hollis BW, Ambs A, Ulrich CM, McTiernan A, Bernstein L, Wayne S, Gilliland F, Baumgartner K, Baumgartner R, Ballard-Barbash R (2008) Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr 88 (1): 133–139.
Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ, Dunstan CR (2010) Vitamin D deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone 47 (4): 795–803.
Ordonez-Mena JM, Schottker B, Haug U, Muller H, Kohrle J, Schomburg L, Holleczek B, Brenner H (2013) Serum 25-hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev 22 (5): 905–916.
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6 (1): 40–57.
Rejnmark L, Tietze A, Vestergaard P, Buhl L, Lehbrink M, Heickendorff L, Mosekilde L (2009) Reduced prediagnostic 25-hydroxyvitamin D levels in women with breast cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev 18 (10): 2655–2660.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371 (9612): 569–578.
Robien K, Cutler GJ, Lazovich D (2007) Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women's Health Study. Cancer Causes Control 18 (7): 775–782.
Robsahm TE, Tretli S, Dahlback A, Moan J (2004) Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 15 (2): 149–158.
Rose AA, Elser C, Ennis M, Goodwin PJ (2013) Blood levels of vitamin D and early stage breast cancer prognosis: a systematic review and meta-analysis. Breast Cancer Res Treat 141 (3): 331–339.
Scarmo S, Afanasyeva Y, Lenner P, Koenig KL, Horst RL, Clendenen TV, Arslan AA, Chen Y, Hallmans G, Lundin E, Rinaldi S, Toniolo P, Shore RE, Zeleniuch-Jacquotte A (2013) Circulating levels of 25-hydroxyvitamin D and risk of breast cancer: a nested case-control study. Breast Cancer Res 15 (1): R15.
Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC (2002) Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst 94 (17): 1301–1311.
So JY, Lee HJ, Smolarek AK, Paul S, Wang CX, Maehr H, Uskokovic M, Zheng X, Conney AH, Cai L, Liu F, Suh N (2011) A novel Gemini vitamin D analog represses the expression of a stem cell marker CD44 in breast cancer. Mol Pharmacol 79 (3): 360–367.
Sperati F, Vici P, Maugeri-Saccà M, Stranges S, Santesso N, Mariani L, Giordano A, Sergi D, Pizzuti L, Di Lauro L, Montella M, Crispo A, Mottolese M, Barba M (2013) Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PLoS One 8 (7): e69269.
Steindorf K, Ritte R, Eomois PP, Lukanova A, Tjonneland A, Johnsen NF, Overvad K, Ostergaard JN, Clavel-Chapelon F, Fournier A, Dossus L, Teucher B, Rohrmann S, Boeing H, Wientzek A, Trichopoulou A, Karapetyan T, Trichopoulos D, Masala G, Berrino F, Mattiello A, Tumino R, Ricceri F, Quiros JR, Travier N, Sanchez MJ, Navarro C, Ardanaz E, Amiano P, Bueno-de-Mesquita HB, van Duijnhoven F, Monninkhof E, May AM, Khaw KT, Wareham N, Key TJ, Travis RC, Borch KB, Sund M, Andersson A, Fedirko V, Rinaldi S, Romieu I, Wahrendorf J, Riboli E, Kaaks R (2013) Physical activity and risk of breast cancer overall and by hormone receptor status: the European prospective investigation into cancer and nutrition. Int J Cancer 132 (7): 1667–1678.
Stoica A, Saceda M, Fakhro A, Solomon HB, Fenster BD, Martin MB (1999) Regulation of estrogen receptor-alpha gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem 75 (4): 640–651.
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283 (15): 2008–2012.
Sundaram S, Sea A, Feldman S, Strawbridge R, Hoopes PJ, Demidenko E, Binderup L, Gewirtz DA (2003) The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res 9 (6): 2350–2356.
Swami S, Krishnan AV, Feldman D (2000) 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res 6 (8): 3371–3379.
Swami S, Krishnan AV, Wang JY, Jensen K, Horst R, Albertelli MA, Feldman D (2012) Dietary vitamin D(3) and 1,25-dihydroxyvitamin D(3) (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 153 (6): 2576–2587.
Tirona MT, Sehgal R, Ballester O (2010) Prevention of breast cancer (Part I): epidemiology, risk factors, and risk assessment tools. Cancer Invest 28 (7): 743–750.
Tretli S, Schwartz GG, Torjesen PA, Robsahm TE (2012) Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: a population-based study. Cancer Causes Control 23 (2): 363–370.
Vaidya A, Williams JS, Forman JP (2012) The independent association between 25-hydroxyvitamin D and adiponectin and its relation with BMI in two large cohorts: the NHS and the HPFS. Obesity (Silver Spring) 20 (1): 186–191.
Villasenor A, Ballard-Barbash R, Ambs A, Bernstein L, Baumgartner K, Baumgartner R, Ulrich CM, Hollis BW, McTiernan A, Neuhouser ML (2013) Associations of serum 25-hydroxyvitamin D with overall and breast cancer-specific mortality in a multiethnic cohort of breast cancer survivors. Cancer Causes Control 24 (4): 759–767.
Vrieling A, Seibold P, Johnson TS, Heinz J, Obi N, Kaaks R, Flesch-Janys D, Chang-Claude J (2013) Circulating 25-hydroxyvitamin D and postmenopausal breast cancer survival: Influence of tumor characteristics and lifestyle factors? Int J Cancer e-pub ahead of print 22 November 2013 doi:10.1002/ijc.28628.
Wang D, Dubois RN (2004) Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol 31 (1 Suppl 3): 64–73.
Wang D, Velez de-la-Paz OI, Zhai JX, Liu DW (2013) Serum 25-hydroxyvitamin D and breast cancer risk: a meta-analysis of prospective studies. Tumor Biol 34 (6): 3509–3517.
Welsh J (2004) Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr 80 (6 Suppl): 1721S–1724S.
World Cancer Research Fund (2010) Breast Cancer 2010 report: food, nutrition, physical activity, and the prevention of breast cancer. World Cancer Research Fund/American Institute of Cancer Research.
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72 (3): 690–693.
Wu Y, Zhang D, Kang S (2013) Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 137 (3): 869–882.
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H (2010) Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 46 (12): 2196–2205.
Zehnder D, Bland R, Williams M, McNinch RW, Howie AJ, Stewart PM, Hewison M (2001) Extrarenal expression of 25-hydroxyvitamin D(3)-1a-hydroxylase. J Clin Endocrinol Metab 86: 888–894.
Acknowledgements
Author contributions
All authors have read and approved the final version submitted for publication. YJ developed study concept and design and contributed to critical revision of the manuscript for important intellectual content; YK wrote the manuscript; YK and YJ researched data, conducted the statistical analysis, contributed to discussion and reviewed/edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kim, Y., Je, Y. Vitamin D intake, blood 25(OH)D levels, and breast cancer risk or mortality: a meta-analysis. Br J Cancer 110, 2772–2784 (2014). https://doi.org/10.1038/bjc.2014.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2014.175
Keywords
This article is cited by
-
Evaluating the effect of vitamin D supplementation on serum levels of 25-hydroxy vitamin D, 1,25-dihydroxy vitamin D, parathyroid hormone and renin–angiotensin–aldosterone system: a systematic review and meta-analysis of clinical trials
BMC Nutrition (2023)
-
Serum 25-hydroxyvitamin D and cancer-related fatigue: associations and effects on depression, anxiety, functional capacity and health-related quality of Life in breast cancer survivors during adjuvant endocrine therapy
BMC Cancer (2022)
-
Melatonin and vitamin D: complementary therapeutic strategies for breast cancer
Supportive Care in Cancer (2021)
-
The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis
Breast Cancer Research and Treatment (2020)
-
25-Hydroxyvitamin D at time of breast cancer diagnosis and breast cancer survival
Breast Cancer Research and Treatment (2020)