Abstract
Background:
The clinical significance of circulating tumour cell (CTC) detection in gastrointestinal (GI) cancer remains controversial and the molecular biological characteristics of CTCs are poorly understood.
Methods:
A total of 87 patients with metastatic or recurrent GI cancer were prospectively enrolled. Circulating tumour cells and their HER2 status were assessed using the CellSearch System.
Results:
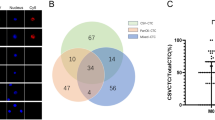
Among the 62 CTC-positive cases, we found 22 discordant cases (35.5%). Among the HER2-negative primary tumours, 17 of 54 developed HER2-positive CTCs. Five of eight had HER2-negative CTCs among the HER2-positive primary tumours.
Conclusion:
The findings in the current study suggest that it is critical to evaluate the HER2 status of not only the primary tumour but also the CTCs because the metastasising tumour cells are the primary target of systemic therapy.
Similar content being viewed by others
Main
Tumour recurrence is often observed in patients with gastrointestinal (GI) cancers even after a complete resection, indicating that undetectable tumour deposits must be present at the time of surgery. Circulating tumour cells (CTCs) can be released from the primary tumour into the blood stream and colonise distant organs giving rise to metastasis. The FDA-approved CellSearch System (Veridex, Raritan, NJ, USA) is a semi-automated analyser enriching CTCs with ferrofluid nanoparticles coated with anti-EpCAM antibodies. This system has proved to be useful for detecting and analysing CTCs in patients with breast, colorectal or prostate cancer (Negin and Cohen, 2010).
Gastrointestinal cancers are among the most diagnosed of all solid tumours. Although the development of multidisciplinary treatments (such as surgical treatment and chemotherapy) has improved the prognosis of GI cancer, the overall survival of the patients with metastatic or recurrent disease remains dismal. Recently, molecularly targeted therapy has been used in GI cancer, leading to significant improvements in overall survival (Van Cutsem et al, 2009; Bang et al, 2010; Douillard et al, 2010). Some monoclonal antibodies and kinase inhibitors, targeting EGFR and VEGF/VEGFR, have demonstrated promising efficacy in GI cancer. The ToGA trials revealed the addition of the anti-HER2 agent trastuzumab to chemotherapy in HER2-positive patients significantly improved the overall survival in gastric cancer (Bang et al, 2010). In a clinical setting, the evaluation of HER2 status is restricted to the primary tumour. However, it is reported that assessment of the HER2 status in tissue biopsies carries a significant risk of sampling errors, thereby rendering patients unsuitable for treatment with trastuzumab (Warneke et al, 2012). Because the metastasising tumour cells are the primary targets of systemic therapy, it is critical to confirm the expression of HER2 in CTCs. In addition, it is possible that the treatment strategy could be affected by the HER2 status of CTCs.
In the current study, the aims were to elucidate the HER2 status of CTCs in metastatic or recurrent GI cancer at the time of diagnosis by use of the CellSearch System and to assess the correlation of HER2 status between primary tumour and corresponding CTCs.
Materials and methods
Patients
Between 2008 and 2012, 87 patients with metastatic or recurrent GI cancer (oesophageal, 20; gastric, 34; and colorectal, 33 cases) were prospectively enrolled. All patients were histopathologically positive for GI cancer. Documented informed consent was obtained from all patients, and the study protocol was approved by the local ethics committee.
Clinical samples
For measurement of CTCs, pre-treatment peripheral blood (PB) samples (7.5 ml) were collected in CellSave tubes at Kumamoto University. All PB samples were immediately transferred to Kanagawa and measured within 48 h. For the patients with distant metastasis at the time of diagnosis, biopsy samples were routinely obtained from the primary tumour through GI endoscopy and made available for the evaluation of HER2 status (oesophageal, 19; gastric, 25; and colorectal, 20 cases). At least three biopsy fragments were assessed in each cases. For the patients with recurrent disease after curative resection, resected specimens of the primary tumour were available for the evaluation of HER2 status (oesophageal, 1; gastric, 9; and colorectal, 13 cases). Normal, negative controls consisted of PB samples that were collected from 10 volunteers lacking malignancies.
CTC measurement
Peripheral blood samples were enriched with the CellSearch System with ferrofluid nanoparticles coated with anti-EpCAM antibody. The enriched EpCAM+ population was stained with phycoerythrin-conjugated antibodies against CK8, 18 and 19, with allophycocyanin-conjugated antibodies specific for leucocytes (anti-CD45 antibody) and with the nuclear dye 4’, 6-diamino-2-phenylidole (DAPI) for nucleic acid staining. The CK+/DAPI+/CD45− cells were counted as CTCs using a CellTracks Analyzer II (Veridex), a four-colour semi-automated fluorescent microscope (Allard et al, 2004).
Determination of HER2 status of CTCs and primary tumours
The HER2 status of CTCs was assessed with the fluorescein-labelled anti-HER2 antibody (CellSearch System Tumour Phenotyping Reagent HER2) as previously reported (Riethdorf et al, 2010). As shown in Figure 1, HER2 staining of CTCs from GI cancer patients observed in the FITC channel of the CellSearch System was classified as positive or negative in this study as with previous study by Riethdorf et al (2010).
The HER2 status of primary tumours was assessed by immunohistochemical study (HercepTest, Dako, Glostrup, Denmark). The scoring criteria were based on the study by Ruschoff et al (2010) and tumours were classified as 0, 1+, 2+ and 3+.
Statistical analysis
For continuous variables, data were expressed as means±standard deviation and compared by the Mann–Whitney test. Association between categorical variables was analysed using a chi-square test and Student’s t-test. Findings were considered significant when the P-value was <0.05. All tests were performed using JMP software (SAS Institute Inc., Cary, NA, USA).
Results
Patient characteristics
A total of 87 patients with metastasis (61 patients) and recurrence (26 patients) and ten healthy volunteers were enrolled in this study. The patient characteristics are summarised in Table 1. The patterns of metastasis or recurrence included local (8 patients), distant lymph node (10 patients), hematogenous organs such as lung, liver and bone (52 patients) and peritoneal dissemination (17 patients).
Detection of CTCs in GI cancers
The CellSearch System was used to analyse a total of 87 PB samples from all GI cancers. At least one CTC was detected in 62 samples (71.3%). The mean number of CTCs was 117 (0–4800 cells). No CTCs were detected in any of the samples from healthy volunteers. At least one CTC was detected in 15 of 20 samples (75.0%) in oesophageal cancer, 25 of 34 samples (73.5%) in gastric cancer and 22 of 33 samples (66.6%) in colorectal cancer. The mean number of CTCs was 201 (0–4800 cells), 104 (0–2756 cells) and 18.4 (0–353 cells) in oesophageal, gastric and colorectal cancer, respectively.
HER2 status of CTCs in GI cancers and comparison of HER2 status between CTCs and primary tumours
Among the 62 CTC-positive cases, HER2-positive CTCs were detected in 20 (32.3%) cases (6 of 15 oesophageal cancer (40.0%), 7 of 25 gastric cancer (28.0%) and 7 of 22 colorectal cancer (31.8%)). Next, we compared the HER2 status between CTCs and corresponding primary tumours in 62 CTC-positive cases (Table 2). We found 22 (35.5%) discordant cases (HER2-positive CTCs/HER2-negative in primary tumour 17 of 54 (31.4%), HER2-negative CTCs/HER2-postive in primary tumour 5 of 8 (62.5%). There was no significant correlation of HER2 status between CTCs and primary tumour in all types of cancer.
Discussion
In this study, we revealed the frequency of HER2 expression in CTCs in patients with metastatic or recurrent GI cancer. HER2-positive CTCs were observed in 32.3% of all GI cancer patients. Circulating tumour cells were particularly evident in the patients with hemtogenous metastasis (59.8%). The presence of HER2-positive CTCs has been analysed in breast and bladder cancer (Pestrin et al, 2009; Fehm et al, 2010; Ignatiadis et al, 2011; Rink et al, 2012). Riethdorf et al (2010) revealed that HER2 over-expression in CTCs in breast cancer patients was associated with high tumour stage and suggested that determining HER2 status of CTCs could help identify patients who might benefit from trastuzumab-containing regimens in a neoadjuvant setting. Therefore, identification of the HER2 status of CTCs is a logical step because CTCs colonise distant organs, giving rise to metastasis. In fact, a clinical trial testing the use of lapatinib in advanced breast cancer with HER2-negative in primary tumours and HER2-positive or EGFR-positive CTCs was recently completed (clinicaltrials.gov/:NCT00820924). The EORTC group is also planning to launch the ‘Treat CTC’ trial. Treat CTC will investigate whether trastuzumab can reduce CTC detection and disease recurrences compared with observation in patients with HER2-negative breast cancer.
There are some limitations concerning about the evaluation of HER2 expression in this study. In our series, we found 22 (35.5%) discordant cases in CTC-positive cases. Among the HER2-negative primary tumours, 31.4% (17 of 54) developed HER2-positive CTCs. Inversely, 62.5% (5 of 8) had HER2-negative CTCs among the HER2-positive primary tumours. No concordance of HER2 status between primary tumour and CTCs was observed in this study as previous several studies (Riethdorf et al, 2010; Ignatiadis et al, 2011). There are some possible reasons for discordant HER2 status. First, it is possible that HER2 status of a primary tumour evaluated by biopsy might not reflect the HER2 status of the whole primary tumour. Gastric cancer is well known to have histological heterogeneity, various histological types and differentiation are frequently observed in the same specimen. On the other hand, Shinozaki et al (2012) reported high concordance of HER2 expression between surgically resected specimens and prior biopsy specimens in the HER2-positive group. However, at least three fragments seemed to be required to assess precisely to avoid false-negative results (Shinozaki et al, 2012). Therefore, we assessed at least three biopsy fragments to avoid false-negative result. Second, unlike breast and gastric cancer, the methods for evaluating HER2 status in resected or biopsy specimens have not been established in oesophageal and colorectal cancer. As a result, the frequency of HER2-positive expression in GI cancer varies in several reports (Nathanson et al, 2003; Khan et al, 2006). Third, it is possible that some cancer patients whose primary tumour was HER2-negative acquired HER2 gene amplification in their CTCs during cancer progression as previously reported (Meng et al, 2004). In this study, only samples obtained at diagnosis before treatment were collected to avoid transition of HER2 status. Furthermore, the discordant of HER2 status seems to be observed in both direction. Interestingly, some cases in HER2-negative CTCs/HER2-positive primary tumours group have close cut-off value of HER2 copy number evaluated by FISH. Therefore, it is critical to evaluate the HER2 status of CTCs because the metastasising tumour cells are the primary target of systemic therapy.
In conclusion, we clarified the frequency of HER2-positive CTCs in metastatic or recurrent GI cancer. The information on the HER2 status of CTC might be helpful for stratification of HER2-directed therapy. More studies are urgently required to test the clinical utility of using CTCs as a liquid biopsy to monitor tumour genotypes and to contribute to personalised treatment strategies in GI cancer.
Change history
26 November 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW (2004) Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 10: 6897–6904.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocakova I, Ruff P, Blasinska-Morawiec M, Smakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28: 4697–4705.
Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, StickeleR E, Lattrich C, Lohberg CR, Solomayer E, Rack B, Riethdorf S, Klein C, Schindlbeck C, Brocker K, Kasimir-Bauer S, Wallwiener D, Pantel K (2010) HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 124: 403–412.
Ignatiadis M, Rothe F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, Metallo J, Kheddoumi N, Singhal SK, Michiels S, Veys I, Rossari J, Larsimont D, Carly B, Pestrin M, Bessi S, Buxant F, Liebens F, Piccart M, Sotiriou C (2011) HER2-positive circulating tumor cells in breast cancer. PLoS One 6: e15624.
Khan AN, Yang W, Seifalian AM, Winslet MC (2006) HER2 (ErbB2) receptors, a potential therapeutic target in squamous cell carcinoma of oesophagus. Br J Cancer 94: 1213–1214.
Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, Frenkel E, Hoover S, Leitch M, Clifford E, Vitetta E, Morrison L, Herlyn D, Terstappen LW, FLEMING T, Fehm T, Tucker T, Lane N, Wang J, Uhr J (2004) HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA 101: 9393–9398.
Nathanson DR, Culliford ATT, Shia J, Chen B, D'Alessio M, Zeng ZS, Nash GM, Gerald W, Barany F, Paty PB (2003) HER 2/neu expression and gene amplification in colon cancer. Int J Cancer 105: 796–802.
Negin BP, Cohen SJ (2010) Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol 11: 1–13.
Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, Cappadona S, Biganzoli L, Giannini A, Di Leo A (2009) Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 118: 523–530.
Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I, Hilfrich J, Holms F, Tesch H, Eidtmann H, Untch M, Von Minckwitz G, Pantel K (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16: 2634–2645.
Rink M, Chun FK, Dahlem R, Soave A, Minner S, Hansen J, Stoupiec M, Coith C, Kluth LA, Ahyai SA, Friedrich MG, Shariat SF, Fisch M, Pantel K, Riethdorf S (2012) Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol 61: 810–817.
Ruschoff J, Dietel M, Baretton G, Arbogast S, Walch A, Monges G, Chenard MP, Penault-Llorca F, Nagelmeier I, Schlake W, Hofler H, Kreipe HH (2010) HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 457: 299–307.
Shinozaki E, Yamamoto N, Chin K, Ogura M, Suenaga M, Matsusaka S, Mizunuma N, Toshiharu Y, Hatake K (2012) How many biopsy fragments will be necessary to assess HER2 status for gastric cancer? J Clin Oncol 30 (suppl 4): abstr 40.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360: 1408–1417.
Warneke VS, Behrens HM, Boger C, Becker T, Lordick F, Ebert MP, Rocken C (2012) Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol. 24 (3): 725–733.
Acknowledgements
We thank Mr K Miyake and Ms Yokoyama for their excellent technical assistance. This work was supported by the following grants: Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research; grant number 25462027.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Iwatsuki, M., Toyoshima, K., Watanabe, M. et al. Frequency of HER2 expression of circulating tumour cells in patients with metastatic or recurrent gastrointestinal cancer. Br J Cancer 109, 2829–2832 (2013). https://doi.org/10.1038/bjc.2013.680
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.680
Keywords
This article is cited by
-
Circulating tumor cells in gastric cancer: developments and clinical applications
Clinical and Experimental Medicine (2023)