Abstract
Background:
This study investigated the usefulness of a novel inflammation-based prognostic system, named the COP-NLR (COmbination of Platelet count and Neutrophil to Lymphocyte Ratio), for predicting the postoperative survival of patients with colorectal cancer (CRC).
Methods:
The COP-NLR was calculated on the basis of data obtained on the day of admission: patients with both an elevated platelet count (>30 × 104 mm−3) and an elevated NLR (>3) were allocated a score of 2, and patients showing one or neither were allocated a score of 1 or 0, respectively.
Results:
Four-hundred and eighty patients were enrolled. Multivariate analysis of clinical characteristics selected by univariate analysis showed that the COP-NLR (1, 2/0) (odds ratio, 0.464; 95% confidence interval, 0.267–0.807; P=0.007) had an association with cancer-specific survival, along with pathology, lymph node metastasis, the serum levels of carcinoembryonic antigen, C-reactive protein and albumin, and the Glasgow Prognostic Score. Kaplan–Meier analysis and log-rank test revealed that the COP-NLR was able to divide such patients into three independent groups (P<0.001).
Conclusion:
The COP-NLR is considered to be a useful predictor of postoperative survival in patients with CRC.
Similar content being viewed by others
Main
Recent studies have demonstrated that the systemic inflammatory response (SIR) (McMillan et al, 2003) is associated with postoperative survival in patients with several types of cancer (Forrest et al, 2004; Ishizuka et al, 2007; Ramsey et al, 2007). Although there are a number of inflammation-based prognostic systems and clinical measures based on SIR, such as the Glasgow Prognostic Score (GPS) (Forrest et al, 2004; Ramsey et al, 2007; Ishizuka et al, 2012), neutrophil to lymphocyte ratio (NLR) (Bhatti et al, 2010; Kim et al, 2010; Shimada et al, 2010; Chua et al, 2011), and reactive thrombocytosis (Silvis et al, 1970; Monreal et al, 1998; Shimada et al, 2004), their systems of evaluation differ. Although the GPS is based on estimation of two types of protein – the serum levels of C-reactive protein (CRP) and albumin, which are regulated by inflammatory cytokines – the NLR and thrombocytosis are based on cellular components that are also regulated by such cytokines, especially interleukin-6 (IL-6) (Ohsugi, 2007).
Among the inflammatory cytokines, IL-6 is known to be a multifunctional cytokine that acts on a variety of cells, including immune-competent cells and hematopoietic cells, to trigger proliferation and differentiation. From a clinical viewpoint, it is also important to consider that IL-6 stimulates hepatocytes to induce acute-phase proteins, including CRP, and decrease the serum albumin level (Ramadori et al, 1998; Ohsugi, 2007). This phenomenon is merely part of the mechanism reflected in the GPS. Similarly, IL-6 elicits not only neutrophil proliferation but also differentiation of megakaryocytes to platelets (Imai et al, 1991; Ruscetti, 1994), and these phenomena are also related to the mechanisms underlying SIR.
There is an increasing evidence that the GPS, reactive thrombocytosis, and the NLR can be used for prognostication in patients with several types of cancer (Shimada et al, 2004, 2010). Because various combinations of acute-phase proteins can be employed for this purpose, it would be expected that estimation of reactive thrombocytosis (Shimada et al, 2004) and NLR (Shimada et al, 2010) would also be potentially applicable for prognostication.
In the present study, therefore, we evaluated the prognostic utility of a novel inflammation-based prognostic system, named the COP-NLR (COmbination of Platelet count and Neutrophil to Lymphocyte Ratio), in patients undergoing surgery for colorectal cancer (CRC).
Materials and methods
We conducted a retrospective review of a database comprising 490 patients who had undergone elective surgery for CRC. All procedures had been performed by the same surgical team at the Department of Gastroenterological Surgery, Dokkyo Medical University Hospital, between January 2000 and August 2009. Among these patients, 480 were enrolled in this study on the basis of specific criteria. Patients for whom insufficient laboratory data were available in the record, or those who had idiopathic thrombocytopenic purpura (n=2), were excluded.
Routine laboratory measurements including the serum levels of CRP, albumin, and tumour markers such as carcinoembryonic antigen (CEA; upper physiological value 5 ng ml−1) and carbohydrate antigen 19-9 (upper physiological value 37 U ml−1) were carried out on the day of admission in order to exclude any effects attributable to inflammation associated with sequential preoperative examinations. None of the patients had clinical evidence of infection or other inflammatory conditions, and none had received preoperative chemotherapy or irradiation.
The recommended cut-off values of the preoperative NLR and platelet count were decided using receiver operating characteristic (ROC) curve analyses. The recommended cut-off value of the NLR was based on the most prominent point on the ROC curve for sensitivity (0.492) and specificity (0.742), respectively. Because these two parameters indicated a cut-off value of 2.902, the recommended NLR cut-off value was defined as 3.0. The area under the ROC curve was 0.619. Similarly, the recommended cut-off value of the preoperative platelet count was defined as 30 × 104 mm−3, because the most prominent point on the ROC curve indicated a cut-off value of 28.85 × 104 mm−3 for sensitivity (0.413) and specificity (0.742). The area under the ROC curve was 0.589.
The COP-NLR was calculated on the basis of data obtained on the day of admission as follows: patients with both an elevated platelet count (>30 × 104 mm−3) and an elevated NLR (>3) were allocated a score of 2, and patients showing one or neither were allocated a score of 1 or 0, respectively.
Univariate analysis was performed to evaluate clinical characteristics including age (year), sex (male/female), number of tumours (⩾2/1), maximum tumour diameter (>40/⩽40 mm), tumour location (rectum/colon), tumour type (3, 4, and 5/0, 1, and 2), pathology (others/tub1, 2), lymphatic invasion (presence/absence), venous invasion (presence/absence), lymph node metastasis (presence/absence), white blood cell (WBC) count ( × 103 mm−3), platelet count ( × 104 mm−3), neutrophil ratio (%), lymphocyte ratio (%), NLR, the serum levels of CRP (mg dl−1), albumin (g dl−1), CEA (ng ml−1), and CA19-9 (U ml−1), body mass index (BMI) (kg m−2), the GPS (2/0, 1), and the COP-NLR (1, 2/0) relative to cancer-specific survival.
Multivariate analysis was then performed using clinical characteristics with a P-value <0.05 selected in the univariate analysis to assess those that were predictive of cancer-specific survival.
Kaplan–Meier analysis and log-rank test were used to compare the survival curves of the three groups divided according to the COP-NLR scores of 0, 1, and 2.
Estimation of the GPS
The GPS was estimated as described previously. Briefly, patients with both an elevated CRP level (>1.0 mg dl−1) and hypoalbuminemia (<3.5 g dl−1) were allocated a score of 2. Patients in whom only one of these biochemical abnormalities was present were allocated a score of 1, and those in whom neither of these abnormalities was present were allocated a score of 0 (Forrest et al, 2004).
Definition of operative curability
Based on the Japanese Classification of Colorectal Carcinoma (Japanese Society for Cancer of the Colon and Rectum, Second English Edition), residual tumour is diagnosed as follows: R0, no residual tumour; R1, no residual tumour, but tumour suspected at the resection margin; and R2, macroscopically evident residual tumour. Based on this definition, operative curability is defined as follows: curability A, R0 in TNM stage I, II, or III; curability B, R0 in TNM stage IV or R1 in any TNM stage; curability C, R2 in any TNM stage.
Definition of macroscopic tumour types and histological findings
Similarly, macroscopic tumour types are classified as follows: type 0, superficial; type 1, polypoid; type 2, ulcerated with clear margin; type 3, ulcerated with infiltration; type 4, diffusely infiltrating; and type 5, unclassified. On the basis of these definitions, we classified the patients into two groups: those with noninvasive tumours (types 0, 1, and 2) and those with invasive tumours (types 3, 4, and 5).
The pathological types of tumours are defined as follows: tub1, well-differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma; por, poorly differentiated adenocarcinoma; muc, mucinous adenocarcinoma; and sig, signet ring cell carcinoma. On the basis of these definitions, the patients were divided into two groups: those with differentiated adenocarcinoma (tub1 and tub2) and those with other types of carcinoma (por, muc, and sig).
Invasion of vessels – that is, lymphatic invasion (ly) and venous invasion (v) – was diagnosed as follows: ly0 (v0), no invasion; ly1 (v1), minimal invasion; ly2 (v2), moderate invasion; and ly3 (v3), severe invasion. On the basis of these definitions, the patients were divided into two groups: those without (ly0, v0) and those with (ly1–3, v1–3) vessel invasion.
Administration of chemotherapy
Most of the stage IV patients undergoing surgery were considered for postoperative chemotherapy. Because, in our department, recently developed chemotherapy regimens such as FOLFIRI (FOLinic acid (leucovorin)/Fluorouracil (5-FU)/IRInotecan (Camptosar)) (Tournigand et al, 2004) and FOLFOX (FOLinic acid (leucovorin)/Fluorouracil (5-FU)/OXaliplatin (Eloxatin)) (de Gramont et al, 2000) were introduced in January 2005, patients who had undergone surgery before January 2005 had been administered oral anticancer drugs based on 5-FU as postoperative chemotherapy (Hernandez et al, 1992).
Similarly, most of the stage III patients undergoing surgery were considered for postoperative chemotherapy. Because, in our department, recently developed chemotherapy regimens such as capecitabine (Twelves et al, 2005) were introduced in October 2008, patients who had undergone surgery before October 2008 were administered the same drugs as those for stage IV patients (Kaser et al, 2001). In addition, most of the stage II patients undergoing surgery were not considered for postoperative chemotherapy.
Statistical analysis
Data are presented as mean±s.d. Differences among the three groups were analysed using the χ2- test and the Kruskal–Wallis test. Odds ratios (OR) with 95% confidence interval (CI) were calculated by uni- or multivariate Cox proportional hazards model analyses.
Kaplan–Meier analysis and log-rank test were used to compare survival curves. Deaths before 31 March 2009 were included in this analysis.
Statistical analyses were performed using the SPSS statistical software package, version 16.0 (SPSS Inc., Chicago, IL, USA) at a significance level of P<0.05.
Results
A total of 480 patients were enrolled (male : female=309 (64.4%) : 171 (35.6%)). There were 356 (74.2%) patients with a platelet count of ⩽30 × 104 mm−3 and 124 (25.8%) patients with a platelet count of >30 × 104 mm−3. Among these patients, 338 (70.4%) patients had a NLR of ⩽3 and 142 (29.6%) patients had a NLR of >3.
Table 1 shows the distribution of the clinical background characteristics of the studied patients in the three groups divided according to the COP-NLR. There were no significant differences among the groups, except for maximum tumour diameter (⩽40/>40, mm) (P<0.001), tumour type (0, 1, 2/3, 4, and 5) (P=0.016), lymphatic invasion (absence/presence) (P<0.001), venous invasion (absence/presence) (P=0.004), operative curability (A/B/C) (P<0.001), GPS (0/1/2) (P<0.001), and TNM stage (0/I/II/III/IV) (P<0.001) (χ2-test).
Table 2 shows the relationships between clinicolaboratory characteristics and the three groups of patients. There were no significant inter-group differences, except for maximum tumour diameter (mm; P<0.001), WBC count (P<0.001), platelet count (P<0.001), neutrophil ratio (P<0.001), lymphocyte ratio (P<0.001), NLR (P<0.001), the serum levels of CRP (mg dl−1; P<0.001), albumin (g dl−1; P<0.001) and CEA (ng ml−1; P<0.001), BMI (kg m−2; P=0.002), and survival period (P=0.001) (Kruskal–Wallis test).
During the observation period, 150 (31.3%) patients died, among whom 30 (20.0%) died of intercurrent disease. Univariate and multivariate analyses were performed to evaluate the relationship between clinical characteristics and cancer-specific survival.
The results of univariate analyses demonstrated that maximum tumour diameter (⩽40/>40 mm) (OR, 0.517; 95% CI, 0.349–0.767; P=0.001), pathology (others/tub1, 2) (OR, 0.338; 95% CI, 0.205–0.558; P<0.001), lymphatic invasion (presence/absence) (OR, 0.167; 95% CI, 0.068–0.409; P<0.001), venous invasion (presence/absence) (OR, 0.376; 95% CI, 0.207–0.683; P=0.001), lymph node metastasis (presence/absence) (OR, 0.299; 95% CI, 0.201–0.444; P<0.001), WBC count ( × 103 mm−3) (OR, 1.108; 95% CI, 1.060–1.159; P<0.001), platelet count ( × 104 mm−3) (OR, 1.027; 95% CI, 1.011–1.043; P=0.001), neutrophil ratio (%) (OR, 1.041; 95% CI, 1.024–1.059; P<0.001), lymphocyte ratio (%) (OR, 0.951; 95% CI, 0.933–0.970; P<0.001), NLR (OR, 1.126; 95% CI, 1.074–1.179; P<0.001), the serum levels of CRP (mg dl−1) (OR, 1.224; 95% CI, 1.159–1.292; P<0.001), albumin (g dl−1) (OR, 0.530; 95% CI, 0.394–0.714; P<0.001), CEA (ng ml−1) (OR, 1.001; 95% CI, 1.001–1.002; P<0.001), and CA19-9 (U ml−1) (OR, 1.000; 95% CI, 1.000–1.000; P<0.001), the GPS (2/0, 1) (OR, 0.321; 95% CI, 0.208–0.497; P<0.001), and the COP-NLR (1, 2/0) (OR, 0.349; 95% CI, 0.240–0.507; P<0.001) were associated with cancer-specific survival (Table 3).
Multivariate analysis was performed using the characteristics shown to have statistical significance (P<0.05) by univariate analysis. This indicated that the COP-NLR (1, 2/0) was associated with cancer-specific survival (OR, 0.464; 95% CI, 0.267–0.807; P=0.007), along with pathology (others/tub1, 2) (OR, 0.377; 95% CI, 0.217–0.655; P<0.001), lymph node metastasis (presence/absence) (OR, 0.377; 95% CI, 0.241–0.591; P<0.001), the serum levels of CRP (mg dl−1) (OR, 1.189; 95% CI, 1.081–1.308; P<0.001), albumin (g dl−1) (OR, 0.547; 95% CI, 0.376–0.794; P=0.002), and CEA (OR, 1.001; 95% CI, 1.001–1.001; P<0.001), and the GPS (2/0, 1) (OR, 2.604; 95% CI, 1.242–5.456; P=0.011) (Table 4).
The median and maximum follow-up periods for survivors were 1169 and 3936 days, respectively, and the mean follow-up period was 1326±943 days (mean±s.d.). There were significant differences in the periods after surgery among the COP-NLR 0 (1414±841 days), 1 (1253±1058 days) and 2 (1104±1031 days) groups (mean±s.d., P=0.001, Kruskal–Wallis test).
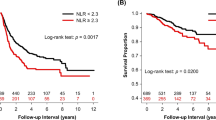
Kaplan–Meier analysis and log-rank test demonstrated that there were significant differences in cancer-specific survival among the three groups (P<0.001) (Figure 1). Thus, the COP-NLR was able to clearly classify such patients into three independent groups.
Discussion
Because the COP-NLR consists of two SIR-related characteristics – NLR and platelet count – it is reasonable that a higher proportion of patients with COP-NLR 2 or 1 would have large tumours, invasive-type tumours, presence of lymphatic invasion, presence of venous invasion, operative curability C, GPS 2, and TNM stage IV than patients with COP-NLR 0. Similarly, the COP-NLR showed close relationships with not only tumour-related characteristics, such as maximum tumour diameter and the serum level of CEA, but also SIR-related characteristics such as WBC count, platelet count, neutrophil ratio, lymphocyte ratio, NLR, the serum levels of CRP and albumin, and BMI. The latter characteristics are related to nutritional status, because an elevated CRP level, hypoalbuminemia, and low BMI reflect cachexia due to hypercytokinemia resulting from tumour progression. These characteristics were retained after uni- and multivariate analyses. Univariate analysis also selected tumour-related characteristics including maximum tumour diameter, pathology, lymphatic invasion, venous invasion, lymph node metastasis, CEA, and CA19-9, and SIR-related characteristics including WBC count, platelet count, neutrophil ratio, lymphocyte ratio, NLR, the serum levels of CRP and albumin, the GPS, and the COP-NLR. Among these clinical characteristics, multivariate analysis disclosed that the COP-NLR was associated with cancer-specific survival, along with not only tumour-related characteristics, such as pathology, lymph node metastasis, and CEA, but also SIR-related characteristics such as CRP, albumin, and the GPS. Therefore, the COP-NLR appears to have potential utility for predicting the postoperative survival of patients with CRC, as well as the GPS.
Recent studies have demonstrated that reactive thrombocytosis is associated with survival after surgery for several types of cancer (Silvis et al, 1970; Monreal et al, 1998; Takahashi et al, 1998; Browder et al, 2000; Shimada et al, 2010). There are two explanations for this phenomenon.
First, reactive thrombocytosis is induced in a background of hypercytokinemia through tumour vs host interaction. Among several inflammatory cytokines, IL-6 has an important role in reactive thrombocytosis (Sierko and Wojtukiewicz, 2004), as it is a multifunctional cytokine with a number of physiological actions, stimulating not only CRP upregulation but also albumin downregulation in the liver, as well as protein synthesis (Ramadori et al, 1998). Similarly, IL-6 has a cell-proliferative effect, triggering the differentiation of megakaryocytes to platelets in the bone marrow (Imai et al, 1991; Ruscetti, 1994). Therefore, it is reasonable that reactive thrombocytosis would be associated with survival after surgery, because this phenomenon is based on the same mechanism as that reflected in the GPS, which is determined from the serum levels of CRP and albumin.
Second, thrombocytosis is also induced from the tumour itself (Mohle et al, 1997; Kohrana and Fine, 2004). Generally, thrombocytosis is a feature in 10–57% of patients with malignancy, as a variety of neoplastic cells can stimulate platelet activation (George et al, 2000; Gunsilius et al, 2000). Several studies have revealed that cancer cells secrete vascular endothelial growth factor (VEGF), which also stimulates megakaryocyte differentiation (Troxler et al, 2007). Because VEGF induction promotes tumour growth (Mohle et al, 1997; Khorana and Fine, 2004; Gorelick et al, 2009), thrombocytosis indirectly reflects tumour progression. In fact, a high level of VEGF is found in serum, platelets, and leukocytes of patients with malignant disease (Lavie et al, 1999), and platelet interactions with malignant cells promote metastasis (Gislason and Nou, 1985).
With regard to the platelet count, most previous studies have used a cut-off value of 30–40 × 104 mm−3 (Symbas et al, 2000; Ishizuka et al, 2012). Although the normal platelet count is 15–30 × 104 mm−3, the cut-off value for reactive thrombocytosis is not clearly defined. Therefore, the cut-off value of the preoperative platelet count used in this study, 30 × 104 mm−3, based on ROC curve analysis, was acceptable.
Previous studies have also demonstrated a relationship between the NLR and postoperative survival in patients with several types of cancer (Shimada et al, 2003; Walsh et al, 2005; Malik et al, 2007; Halazun et al, 2008, 2009; Shimada et al, 2010; Chiang et al, 2012). Because the NLR is based on neutrophils and lymphocytes, it would also be affected by SIR. Although most studies have recommended a NLR cut-off value of 5 (Shimada et al, 2003; Szczepanik et al, 2011), a recent report has recommended a value of 3 (Chiang et al, 2012). Therefore, the NLR cut-off value of 3.0 that we adopted in the present study was acceptable and in line with a previous study that derived a similar value based on ROC curve analysis (Cho et al, 2008).
Shimada et al (2010) have reported that a high NLR was associated with a high platelet count, and selected it as an independent indicator of reduced postoperative survival in patients with gastric cancer. They also reported that not only an elevated CRP level (Shimada et al, 2003) but also reactive thrombocytosis (Shimada et al, 2004) was associated with postoperative survival in patients with oesophageal cancer. These results lend strong support to the use of a combination of reactive thrombocytosis and the NLR for prediction of postoperative survival, along with the GPS.
There are now a number of well-established systemic inflammation-based prognostic scores for patients with CRC. In particular, the GPS and the NLR have been well validated (McMillan, 2013). However, the possibility that additional measures of the SIR might add further significance to these scores has been suggested, as the parameters comprising these scores, that is, CRP, albumin, neutrophils, and lymphocytes, are all correlated with each other (Table 2). To investigate this issue, we examined the ability of neutrophil, lymphocyte, and platelet counts to further discriminate patients in individual groups divided according to whether they had a GPS of 0 or 1, or a GPS of 2. The results obtained from Cox proportional hazards model analyses using the recommended cut-off values from ROC curve analyses revealed that only the platelet count was able to divide both patients with a GPS of 0 or 1, and those with a GPS of 2, into two independent groups (data not shown). These results demonstrated that the platelet count was able to add additional discriminatory ability to not only the GPS but also the NLR; combination of the NLR and the platelet count created a novel inflammation-based prognostic score, the COP-NLR. This is considered to be a meaningful advance in the development of an optimal, routinely available, systemic inflammation-based prognostic score that is able to complement tumour staging.
Theoretically, direct measurement of the serum IL-6 level is the best way to estimate SIR based on tumour vs host interaction. However, there are many unsolved problems associated with the routine measurement of IL-6 in cancer patients. Although a recent study has revealed that the serum level of IL-6 is associated with the postoperative survival of patients with gastric cancer (Szczepanik et al, 2011), routine measurement of IL-6 is difficult in a clinical setting because of its high cost and inconvenience.
On the other hand, the COP-NLR is easy to measure routinely because of its low cost and convenience. In addition, in comparison with the GPS, the COP-NLR may have higher applicability for estimation of SIR, because proliferation and differentiation of cellular components occurs much faster than protein synthesis when inflammatory cytokines are released. Moreover, because repeat measurements of the COP-NLR can be performed more easily, not only before but also after surgery, than those of the GPS and tumour markers, it would deliver solid data based on well-known tumour markers.
Thus, the COP-NLR not only appears capable of classifying patients with CRC into three independent groups before surgery but also has potential as a novel predictor of postoperative survival in such patients.
Change history
23 July 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI (2010) Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 200: 197–203.
Browder T, Folkman J, Pirie-Shepherd S (2000) The hemostatic system as a regulator of angiogenesis. J Biol Chem 275: 1521–1524.
Chiang SF, Hung HY, Tang R, Changchien CR, Chen JS, You YT, Chiang JM, Lin JR (2012) Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int J Colorectal Dis 27: 1347–1357.
Cho S, Cho H, Nam A, Kim HY, Choi YS, Park KH, Cho DJ, Lee BS (2008) Neutrophil-to-lymphocyte ratio as an adjunct to CA-125 for the diagnosis of endometriosis. Fertil Steril 90: 2073–2079.
Chua W, Charles KA, Baracos VE, Clarke SJ (2011) Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 104: 1288–1295.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18: 2938–2947.
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ (2004) Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 90: 1704–1706.
George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI (2000) Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: clinical evidence of platelet scavenging? Clin Cancer Res 6: 3147–3152.
Gislason T, Nou E (1985) Sedimentation rate, leucocytes, platelet count and haemoglobin in bronchial carcinoma: an epidemiological study. Eur J Respir Dis 66: 141–146.
Gorelick C, Andikyan V, Mack M, Lee YC, Abulafia O (2009) Prognostic significance of preoperative thrombocytosis in patients with endometrial carcinoma in an inner-city population. Int J Gynecol Cancer 19: 1384–1389.
Gunsilius E, Petzer A, Stockhammer G, Nussbaumer W, Schumacher P, Clausen J, Gastl G (2000) Thrombocytes are the major source for soluble vascular endothelial growth factor in peripheral blood. Oncology 58: 169–174.
Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP (2008) Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 34: 55–60.
Halazun KJ, Hardy MA, Rana AA, Woodland DC 4th, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr, Emond JC (2009) Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 250: 141–151.
Hernandez E, Lavine M, Dunton CJ, Gracely E, Parker J (1992) Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer 69: 2975–2977.
Imai T, Koike K, Kubo T, Kikuchi T, Amano Y, Takagi M, Okumura N, Nakahata T (1991) Interleukin-6 supports human megakaryocytic proliferation and differentiation in vitro. Blood 78: 1969–1974.
Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K (2007) Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg 246: 1047–1051.
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K (2012) Preoperative thrombocytosis is associated with survival after surgery for colorectal cancer. J Surg Oncol 106: 887–891.
Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H (2001) Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood 98: 2720–2725.
Khorana AA, Fine RL (2004) Pancreatic cancer and thromboembolic disease. Lancet Oncol 5: 655–663.
Kim HS, Han KH, Chung HH, Kim JW, Park NH, Song YS, Kang SB (2010) Neutrophil to lymphocyte ratio for preoperative diagnosis of uterine sarcomas: a case-matched comparison. Eur J Surg Oncol 36: 691–698.
Lavie O, Comerci G, Daras V, Bolger BS, Lopes A, Monaghan JM (1999) Thrombocytosis in women with vulvar carcinoma. Gynecol Oncol 72: 82–86.
Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, Lodge JP, Toogood GJ (2007) Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg 246: 806–814.
McMillan DC (2013) The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39 (5): 534–540.
McMillan DC, Canna K, McArdle CS (2003) Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg 90: 215–219.
Mohle R, Green D, Moore MA, Nachman RL, Rafii S (1997) Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA 94: 663–668.
Monreal M, Fernandez-Llamazares J, Pinol M, Julian JF, Broggi M, Escola D, Abad A (1998) Platelet count and survival in patients with colorectal cancer: a preliminary study. Thromb Haemost 79: 916–918.
Ohsugi Y (2007) Recent advances in immunopathophysiology of interleukin-6: an innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol Pharm Bull 30: 2001–2006.
Ramadori G, Van Damme J, Rieder H, Meyer zum Buschenfelde KH (1998) Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol 18: 1259–1264.
Ramsey S, Lamb GW, Aitchison M, Graham J, McMillan DC (2007) Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer 109: 205–212.
Ruscetti FW (1994) Hematologic effects of interleukin-1 and interleukin-6. Curr Opin Hematol 1: 210–215.
Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Aoki T, Sugaya M, Miyazawa Y, Hayashi H, Miyazaki S, Ochiai T (2003) Elevation of preoperative serum C-reactive protein level is related to poor prognosis in oesophageal squamous cell carcinoma. J Surg Oncol 83: 248–252.
Shimada H, Oohira G, Okazumi S, Matsubara H, Nabeya Y, Hayashi H, Takeda A, Gunji Y, Ochiai T (2004) Thrombocytosis associated with poor prognosis in patients with oesophageal carcinoma. J Am Coll Surg 198: 737–741.
Shimada H, Takiguchi N, Kainuma O, Soda H, Ikeda A, Cho A, Miyazaki A, Gunji H, Yamamoto H, Nagata M (2010) High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 13: 170–176.
Sierko E, Wojtukiewicz MZ (2004) Platelets and angiogenesis in malignancy. Semin Thromb Hemost 30: 95–108.
Silvis SE, Turkbas N, Doscherholmen A (1970) Thrombocytosis in patients with lung cancer. JAMA 211: 1852–1853.
Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA (2000) Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int 86: 203–207.
Szczepanik AM, Scislo L, Scully T, Walewska E, Siedlar M, Kolodziejczyk P, Lenart M, Rutkowska M, Galas A, Czupryna A, Kulig J (2011) IL-6 serum levels predict postoperative morbidity in gastric cancer patients. Gastric Cancer 14: 266–273.
Takahashi Y, Bucana CD, Akagi Y, Liu W, Cleary KR, Mai M, Ellis LM (1998) Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer. Clin Cancer Res 4: 429–434.
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22: 229–237.
Troxler M, Dickinson K, Homer-Vanniasinkam S (2007) Platelet function and antiplatelet therapy. Br J Surg 94: 674–682.
Twelves C, Wong A, Nowacki MP, Abt M, Burris H 3rd, Carrato A, Cassidy J, Cervantes A, Fagerberg J, Georgoulias V, Husseini F, Jodrell D, Koralewski P, Kroning H, Maroun J, Marschner N, Mckendrick J, Pawlicki M, Rosso R, Schuller J, Seitz JF, Stabuc B, Tujakowski J, Van Hazel G, Zaulski J, Scheithauer W (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352: 2696–2704.
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ (2005) Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 91: 181–184.
Acknowledgements
We received no funding/grant support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ishizuka, M., Nagata, H., Takagi, K. et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 109, 401–407 (2013). https://doi.org/10.1038/bjc.2013.350
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.350
Keywords
This article is cited by
-
The platelet pannexin 1-IL-1β axis orchestrates pancreatic ductal adenocarcinoma invasion and metastasis
Oncogene (2023)
-
Prognostic value of pre-operative mean corpuscular volume (MCV) in colorectal cancer
Irish Journal of Medical Science (1971 -) (2023)
-
Neutrophil lymphocyte ratio: a reliable biomarker for diabetic nephropathy?
International Journal of Diabetes in Developing Countries (2022)
-
Comprehensive comparison of the prognostic value of systemic inflammation biomarkers for cancer cachexia: a multicenter prospective study
Inflammation Research (2022)
-
Prognostic value of inflammation-based indices in patients with resected hepatocellular carcinoma
BMC Cancer (2021)