Abstract
Background:
The polypeptide N-acetylgalactosaminyltransferases (GalNAc-Ts) family of enzymes regulates the initial steps of mucin-type O-glycosylation. N-acetylgalactosaminyltransferases might show novel patterns of GalNAc-T glycosylation on tumour-derived proteins, which could influence cancer biology, but its mechanisms are unclear. We investigated the association of GalNAc-T3 and -T6 expressions with clinicopathological features and prognoses of patients with renal cell carcinomas (RCCs).
Methods:
Expressions of GalNAc-T3/6 and cell-adhesion molecules were analysed immunohistochemically in 254 paraffin-embedded tumour samples of patients with RCC.
Results:
Of 138 GalNAc-T3+ cases, 46 revealed significant co-expression with GalNAc-T6. N-acetylgalactosaminyltransferases-3+ expression showed a close relationship to poor clinical performance and large tumour size, or pathologically high Fuhrman’s grading, and presence of vascular invasion and necrosis. The GalNAc-T3-positivity potentially suppressed adhesive effects with a significantly low β-catenin expression. Univariate and multivariate analyses showed the GalNAc-T3+ group, but not the GalNAc-T6+ group, to have significantly worse survival rates.
Conclusion:
N-acetylgalactosaminyltransferases-3 expression independently predicts high-grade tumour and poor prognosis in patients with RCC, and may offer a therapeutic target against RCC.
Similar content being viewed by others
Main
Renal cell carcinoma (RCC) is the most common primary malignancy of the kidney and one of the most lethal genitourinary malignancies (Siegel et al, 2012). Approximately 16 000 new cases of RCC are diagnosed each year; 7500 patients die of it in Japan alone (http://www.ganjoho.jp/professional/index.html, 2012), and it recurs within 5 years in up to 20% of RCC patients, even after curative nephrectomy (Zisman et al, 2002). Metastasis of RCC is primarily responsible for its poor prognosis, although immunotherapy and several targeted agents, including tyrosine kinase inhibitors, prolong survival in certain populations (Motzer et al, 2009). Predicting which patients are prone to develop recurrence and mortality after surgery is therefore critical. Although clinicopathological factors, such as the tumour node metastasis (TNM) stage, Fuhrman’s nuclear grading, Eastern Cooperative Oncology Group performance status (ECOG PS), or tumour necrosis, can indicate RCC prognosis (Finley et al, 2011), molecular and genetic factors have not notably done so; the clinical significance of biological markers is under evaluation. The clinical picture of RCC seems to be strongly determined by the complex interplay of additional cellular alterations, for example, epigenetic modulation of gene expression (Baldewijns et al, 2008).
Malignant transformation and progression in tumours are closely related to alterations in cell–surface carbohydrate antigens (CAs), and with frequent aberrant glycosylation (Brockhausen, 1999). Glycosylation, a major post-translational modification for most secretory and cellular proteins, can alter the physicochemical properties and biological activities of these proteins; in cancer, it can change glycoprotein functions and transform cellular phenotypes.
O-glycosylation encompasses diverse classes of glycoproteins. In mammals, the most common types are mucin-type O-linked glycans, which constitute up to 80% of the total amount of CAs (Brockhausen, 1999; Hollingsworth and Swanson, 2004). Mucin-type O-glycosylation in the Golgi apparatus is initiated by uridine diphosphate (UDP) N-acetyl-α-D-galactosamine polypeptide (GalNAc) N-acetylgalactosaminyltransferases (GalNAc-Ts), which catalyse the transfer of GalNAc from the sugar donor UDP-GalNAc to serine and threonine residues on the protein-synthesising CAs (Schwientek et al, 2002; Ten Hagen et al, 2003). O-linked glycans are aberrantly expressed in many epithelial cancers with the presentation of shortened glycan structures and changes in sialylation patterns, which could affect cell differentiation, adhesion, invasion, and metastasis (Brockhausen, 1999; Schwientek et al, 2002; Ten Hagen et al, 2003). However, they are also involved in other numerous biological functions and processes such as fertilisation, anticoagulation, or inflammatory responses (Dwek, 1996; Rudd et al, 2001). To date, 15 distinct members of the GalNAc-T family have been identified and characterised in mammals; as many as 24 human isozymes of it may exist (Schwientek et al, 2002; Ten Hagen et al, 2003). Interestingly, the GalNAc-Ts show tissue-specific expression and have different (but partly overlapping) kinetic properties and acceptor substrate specificities. In contrast, some of them work in a concerted, hierarchical manner to form O-glycans (Ten Hagen et al, 2003). Several studies have indicated that GalNAc-T3 might be useful in the evaluation of various cancer types, including the colorectal (Shibao et al, 2002), lung (Dosaka-Akita et al, 2002; Gu et al, 2004), gastric (Ishikawa et al, 2004), gallbladder (Miyahara et al, 2004), pancreatic (Yamamoto et al, 2004; Li et al, 2011), prostatic (Landers et al, 2005), and extrahepatic bile duct carcinomas (Inoue et al, 2007). Furthermore, it is possible that GalNAc-Ts expression is not only a cancer-specific event but also found in inflamed tissue.
N-acetylgalactosaminyltransferases-6 shows high homology in DNA and amino-acid sequence to GalNAc-T3 throughout the coding region, with identical organisation for nine conserved intron/exon boundaries in the coding regions, and similar kinetic properties that are distinct from other GalNAc-Ts (Schwientek et al, 2002; Ten Hagen et al, 2003; Li et al, 2011). N-acetylgalactosaminyltransferases-6 could thus also be a marker for aberrant O-linked glycans in carcinogenesis. However, we have seen no studies of possible associations between GalNAc-T3 and/or -T6 expressions in RCCs and their clinicopathological features, including tumour grade or patients’ prognoses.
In the current study, using specific polyclonal antibodies against GalNAc-T3 and -T6, we evaluated expressions of GalNAc-T3/6 in RCC specimens with patients’ clinicopathological data, showing that only GalNAc-T3 was expressed in most human RCCs, especially those with worse ECOG PS, or higher Fuhrman’s grade and vascular invasion (VI). Furthermore, GalNAc-T3+ expression has been significantly associated with a poor outcome.
Materials and methods
Patients and tissue specimens
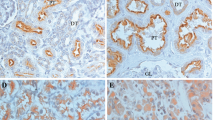
All the intended procedures of the present study, including use of specimens from human subjects, were approved by the Ethics Committee of University of Occupational and Environmental Health (UOEH) in Kitakyushu, Japan. Pathological reports were reviewed to identify patients who underwent radical or partial nephrectomy for RCC between 1999 and December 2011 at the hospital of UOEH. Patients who suffered perioperative deaths, defined as death during the patient’s initial hospitalisation or within 30 days of surgery, were excluded. A total of 254 patients with available follow-up data comprised the cohort of this retrospective study, after further excluding those with the following characteristics: (a) other prior or concomitant malignant tumours; (b) coexisting medical problems of sufficient severity to shorten life expectancy; (c) adjuvant therapies after surgery; and (d) non-carcinomas. Three pathologists examined all resected specimens to confirm their histopathological features. The TNM system of the Union for International Cancer Control (UICC) 7th Edition was used for staging (Sobin et al, 2009); all RCCs were graded based on the Fuhrman four-tiered nuclear grading system (Fuhrman et al, 1982). Clinical information was gathered from patients’ records. The survival duration was based on the date of surgery until patient’s death or most recent clinical visit. Each patient was assigned an ECOG PS score at the time of diagnosis. Patients were categorised as low, intermediate, or high risk, and were prospectively evaluated every 3 months for the first 2 years after surgery, every 6 months for the next 3 years, and yearly thereafter, using chest X-ray or thoracic CT scan, abdominal ultrasonography or CT scan or MRI, and serum biochemistry. A few patients with metastases but without medical contraindications were given palliative interferon-α- and interleukin-2-based immunotherapy. No targeting agents such as tyrosine kinase inhibitors were used. Formalin-fixed, paraffin-embedded tissue blocks came from our Department of Pathology. Normal human tissue was taken from non-tumour portions of surgically resected specimens, and then stained with haematoxylin and eosin, elastica van Gieson (EVG), or immunohistochemical preparations in sequential sections. Elastica van Gieson staining revealed VI very clearly.
Preparation of antibodies against GalNAc-Ts and other Golgi enzymes
Polyclonal antibody was raised against GalNAc-T3 by multiple immunisations of New Zealand white rabbits with a synthetic peptide, based on the previously published work (sequence: GYYTAAELKPVLDRPPQDS; Nomoto et al, 1999). Anti-GalNAc-T6 polyclonal antibody was generated in the same way, as described in the previously published paper (synthetic peptide sequence: GFYTPAELKPFWERPPQDP; Li et al, 2011). The specificity of our two antibodies was confirmed by western blotting and immunohistochemistry, with peptide competition against GalNAc-T3 (Nomoto et al, 1999; Dosaka-Akita et al, 2002) and GalNAc-T6 (Li et al, 2011), respectively. For immunohistochemistry of GalNAc-T3, we used well-differentiated tubular colorectal adenocarcinoma cells as positive controls (Shibao et al, 2002); well-differentiated tubular pancreatic adenocarcinoma cells were used as positive GalNAc-T6 controls (Li et al, 2011).
To analyse staining or expression profiles of other Golgi enzymes or GalNAc-Ts, rabbit polyclonal anti-β-1,2-N-acetylglucosaminyltransferase II (GnT-II), goat polyclonal anti-N-acetylglucosaminyltransferase III (GnT-III), -N-acetylglucosaminyltransferase V (GnT-V), and -Golgi α-mannosidase II (MAN2A1) antibodies (1 : 100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) or anti-GalNAc-T1, -T2, and -T4 goat monoclonal antibodies (1 : 50; Santa Cruz Biotechnology) were applied.
Immunohistochemistry of tissue samples
Immunohistochemical staining was performed by the antibody-linked dextran polymer method (EnVision; DAKO Cytomation, Tokyo, Japan). Deparaffinised and rehydrated 5-μm sections were incubated in 10% H2O2 for 5 min to block endogenous peroxidase activity. The sections were thereafter rinsed and incubated with rabbit polyclonal anti-GalNAc-T3 (diluted 1 : 3000) and -T6 (diluted 1 : 1000), mouse monoclonal β-catenin (diluted 1 : 100, BD Biosciences, San Jose, CA, USA), and mouse monoclonal E-cadherin (diluted 1 : 1000, BD Biosciences) antibodies for 30 min. The second antibody-peroxidase-linked polymers were then applied, and the sections were incubated with a solution consisting of 20 mg of 3.3′-diaminobenzidine tetrahydrochloride, 65 mg of sodium azide, and 20 ml of 30% H2O2 in 100 ml of Tris–HCL (50 mM, pH7.6). After counterstaining with Meyer’s haematoxylin, sections were observed under a light microscope. For immunohistochemistry of RCC tissues, positive areas comprising <10% of the neoplasms were considered negatively stained. For cytoplasmic GalNAc-Ts expressions and apical membranous E-cadherin and β-catenin expressions, respectively, positive areas that were ⩾10% were considered positively stained and were graded into three categories: weak, positive area of 10–30%; moderate, 30–80%; and strong, >80% positive areas. All histological and immunohistochemical slides were evaluated by two independent observers (certified surgical pathologists in our department) using a blind protocol design (observers were blinded to the clinicopathological data). Agreement between observers was excellent (>0.9) for all antibodies investigated, as measured by interclass correlation coefficient. For the few instances of disagreements, a consensus score was determined by the third board-certified pathologists in our department.
Cell culture
Human RCC cell line KPK1 (a kind gift from the Department of Urology, Faculty of Medicine, Kyushu University, Japan) and human lung adenocarcinoma cell line PC9 (Igarashi et al, 2007) were maintained in DMEM and RPMI medium, respectively, containing 10% foetal calf serum (Gibco, Carlsbad, CA, USA) at 37 °C in an atmosphere of 95% air and 5% CO2 (Naito et al, 1982).
Immunofluorescence of RCC tissue samples and cell line KPK1
The frozen sections of human RCC and non-RCC tissue samples were labelled with the above polyclonal rabbit GalNAc-T3 (1 : 3000) and -T6 (1 : 1000) antibodies, polyclonal rabbit GnT-II, polyclonal goat GnT-III, -V, and MAN2A1 (1 : 100; Santa Cruz Biotechnology) antibodies or monoclonal goat GalNAc-T1, -T2, and -T4 (1 : 50; Santa Cruz Biotechnology) antibodies, incubated with Hoechst 33258 (0.5 mg ml−1; Dojindo, Kumamoto, Japan), and then visualised with goat anti-rabbit or donkey anti-goat IgG antibodies conjugated with Alexa Fluor Dyes (green-stained; Invitrogen, Carlsbad, CA, USA).
The RCC cell line KPK1 was cultured on coverslips, fixed with 95% acetone for 5 min, and allowed to air dry. Cells were then incubated with Hoechst 33258 (0.5 mg ml−1; Dojindo) and anti-GalNAc-T3 or -T6 antibodies for 1 h at room temperature (RT), washed with PBS, and reacted with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG for 1 h at RT. After being washed with PBS, specimens were observed under a Nikon (Tokyo, Japan) Eclipse E600 inverted fluorescence microscope.
GalNAc-T3 and GalNAc-T6 siRNA knockdown
Twenty-five base pair double-stranded RNA oligonucleotides were commercially generated (Invitrogen): 5′-AAACAUGUCCAACAACAGAGCAAGG-3′ and 5′-CCUUGCUCUGUUGUUGGACAUGUUU-3′ for GalNAc-T3#84; 5′-UUCUGCUGCUGUAUAAUAUCCUUGC-3′ and 5′-GCAAGGAUAUUAUACAGCAGCAGAA-3′ for GalNAc-T3#85; 5′-UUUCUCUUGGGCCAUCUUUGCUGCC-3′ and 5′-GGCAGCAAAGAUGGCCCAAGAGAAA-3′ for GalNAc-T6#36; and 5′-UUCUCAUGUGGAGGAAGUGUUUCCC-3′ and 5′-GGGAAACACUUCCUCCACAUGAGAA-3′ for GalNAc-T6#38. siRNA transfections were performed according to the manufacturer’s instructions with modifications (Invitrogen; Igarashi et al, 2007; Miyamoto et al, 2008). Five microlitres of Lipofectamine 2000 (Invitrogen) was diluted in 250 μl Opti-MEM medium (Invitrogen) and incubated for 5 min at RT. Next, 250 pmol of GalNAc-T3, GalNAc-T6 and Lipofectamine as control siRNA diluted in 250 μl Opti-MEM were added gently and incubated for 20 min at RT. Oligomer–lipofectamine complexes and aliquots of 2 × 105 PC9 cell (human lung adenocarcinoma cell line; Igarashi et al, 2007) in 500 μl culture medium were combined and incubated for 48 h. Whole-cell extracts (50 μg) were prepared from PC9 after siRNA transfection and western blotting was performed.
Western blotting
The PC9 cells after siRNA transfection were washed and lysed with RIPA buffer. Whole-cell lysates were prepared as previously described (Igarashi et al, 2007). Whole-cell lysates (100 μg) were separated by SDS–polyacrylamide gel electrophoresis and transferred to Immun-Blot polyvinylidene difluoride membranes (Bio-Rad Laboratories, K.K., Tokyo, Japan) using a semi-dry blotter. The blotted membranes were treated with 5% (w/v) skimmed milk in 10 mM Tris, 150 mM NaCl, and 0.2% (v/v) Tween 20, and were incubated for 1 h at RT with the primary antibody. The following antibodies and dilution were used: 1 : 1000 dilution of anti-GalNAc-T3 and anti-GalNAc-T6, and 1 : 5000 dilution of anti-β-actin (Santa Cruz Biotechnology). The membranes were then incubated for 45 min at RT with a peroxidase-conjugated secondary antibody, visualised using an ECL kit (GE Healthcare Bio-Science, Buckinghamshire, UK).
The bands on the western blots were analysed densitometrically using Scion Image software (version 4.0.2; Scion Corp., Frederick, MD, USA).
Statistical analysis
Significance of correlations was calculated by χ2-test to assess relationships between immunohistochemical expressions and clinicopathological variables. Survival curves were plotted using the Kaplan–Meier method and compared with the log-rank test. Hazard ratios and 95% confidence intervals were estimated using univariate or multivariate Cox proportional-hazard models. All statistical tests were two-tailed, with P<0.05 considered significant. All above statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0; Kanda, 2013). More precisely, it is a modified version of R commander (version 1.6-3) designed to add statistical functions frequently used in biostatistics.
Results
Patient characteristics
The cohort included 254 patients (181 men, 73 women) with clinicopathological features representative of RCC (Table 1). The average age at surgery was 63 years. Most patients (191 out of 254) were ECOG 0 patients; the remaining patients (63 out of 254) were ⩾1 PS. The median tumour size was 4.5 cm (range: 1.0–20.0 cm). At diagnosis, 14 patients had lymph node metastases and 34 had distant metastases. Most tumours (218) were classified as clear cell RCC. On the basis of the UICC criteria, 161 patients had stage I disease. Post-operative follow-up was available for all 254 patients (median: 49.0 months; range: 1.0–163.1 months). Median post-operative disease-specific survival (DSS) duration was 36.6 months, with 1- and 5-year survival rates of 96% and 84%, respectively.
GalNAc-Ts or other Golgi enzyme expression in normal tissues and RCC specimens
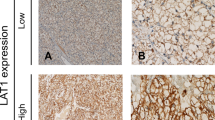
Specificity of GalNAc-T3/6 polyclonal antibodies was tested using immunohistochemistry and western blotting (Nomoto et al, 1999; Dosaka-Akita et al, 2002; Li et al, 2011). N-acetylgalactosaminyltransferases-3 and -6 showed only cytoplasmic immunohistochemical expression (Figure 1). N-acetylgalactosaminyltransferases-3/6 expressions were not detectable or very weakly detectable in adjacent normal tubular epithelium on paraffin-embedded tissues (Figure 1); whereas GalNAc-T3 expression was occasionally and weakly seen in the proximal tubular epithelium on frozen samples, GalNAc-T6 was not (Supplementary Figure 1). N-acetylgalactosaminyltransferases-3 was expressed in 138 of 254 (54%) RCC specimens: 41% weak, 10% moderate, 3% strong. GalNAc-T6 was expressed in 62 of 254 (24%) specimens: 23% weak, 0% moderate, 1% strong. When GalNAc-Ts expression was split into groups of either positive (weak to strong staining) or negative, the GalNAc-T3/6 immunoprofile was 39.4 −/−, 36.2 +/−; 6.3 −/+, and 18.1% +/+.
Representative images of immunohistochemical analyses of GalNAc-T3 and GalNAc-T6 in human RCC (GalNAc-T3: G1–G2, negative; G3: strongly positive; G4: strongly positive; GalNAc-T6: G1–G2, negative; G3, weakly positive; G4: weakly positive) and normal tubule specimens (GalNAc-T3: negative; GalNAc-T6: negative) Original magnification: × 100; inset: × 400. Bar=100 μm ( × 100) or 20 μm ( × 400). Abbreviations: H&E=haematoxylin and eosin; G=Fuhrman’s grade.
Correspondingly, frozen RCC tissue samples showed strong expression of GalNAc-T3 but weak expression of GalNAc-T6 (Supplementary Figure 1), whereas other GalNAc-Ts (-T1, -T2, and -T4) were expressed neither in RCC nor in adjacent normal tubular epithelium on frozen samples (data not shown).
In contrast with the GalNAc-T3 expression profile, frozen RCC specimens demonstrated no expression of other Golgi enzymes, GnT-II and -III, or very weak expression of GnT-V and MAN2A1, despite the specific and functional expression of GnT-II, -III, and -V and MAN2A1 in non-RCC tissues (Supplementary Figure 1). Hence, these obtained data can support that the staining with GalNAc-T3 reflects results of specifically glycosylated structures, but not of merely increased Golgi glycosylation in hyperproliferating RCC cells.
Analysis of GalNAc-Ts expression in human RCC tissues and cell line
Immunofluorescence staining of the RCC surgical specimens and RCC cell line (KPK1) showed specific expression of cytoplasmic GalNAc-T3 in a perinuclear fashion, but very weak expression of cytoplasmic GalNAc-T6 (Supplementary Figures 1 and 2A).
Analysis of specific GalNAc-T3/6 expressions in human cancer cell lines
Western blotting revealed that both proteins of GalNAc-T3 and GalNAc-T6 were expressed in PC9 cell lines. We performed siRNA transfection of GalNAc-T3 or GalNAc-T6 to the wild-type PC9 cells. Expression of GalNAc-T3 was significantly decreased in PC9 cells of GalNAc-T3 siRNA, but not in PC9 cells of GalNAc-T6 siRNA (Supplementary Figure 2B). On the other hand, expression of GalNAc-T6 was significantly downregulated in PC9 cells of GalNAc-T6 siRNA, whereas GalNAc-T6 protein was expressed very well in PC9 cells of GalNAc-T3 siRNA (Supplementary Figure 2B). These western blotting analyses further confirmed that each antibody of GalNAc-T3 and GalNAc-T6 was specific for each protein of GalNAc-T3 and GalNAc-T6, respectively.
Association of GalNAc-T3/6 expression with clinicopathological variables
To identify association of GalNAc-Ts expression (GalNAc-Ts− vs GalNAc-Ts+) with clinicopathological characteristics of the cohort, the variables were split as shown in Table 2. There were no significant differences between patients with GalNAc-T3/6− and GalNAc-T3/6+tumour expressions in terms of age and gender (P>0.05). However, GalNAc-T3+ expression was closely associated with >1 ECOG PS (P=0.02), high tumour Fuhrman’s grade (Figure 1), and advanced disease stage manifesting as larger tumour size and presence of VI and necrosis (P<0.001) in all tumours. GalNAc-T6 stain status also significantly correlated with ECOG PS (P=0.03), tumour Furman’s grade (Figure 1), VI (P<0.001), and necrosis (P=0.01). Particularly, both GalNAc-T3 and -T6 expressions were evident in VI of high-grade RCC components, as shown by EVG stains (Supplementary Figure 3). The high-grade (>G3) tumour rate in GalNAc-T3+ samples was 49 out of 138 (35.5%), but 7 out of 116 (6.0%) in GalNAc-T3− samples. In the GalNAc-T3+ group, 97 out of 138 cases (70.2%) were classified as ⩾stage Ib by UICC TNM classification, compared with 53 out of 116 (45.7%) in the GalNAc-T3− group.
In a Kaplan–Meier analysis (Figure 2), RCC patients with GalNAc-T3/6+ expression had a significantly shorter post-operative median DSS (-T3: 34.5 months; -T6: 34.5 months) compared with those who had GalNAc-Ts− expressions, (-T3: 40.5 months; -T6: 37.0 months; -T3: P<0.001; -T6: P=0.03, Figure 2A and B). For patients with GalNAc-T3+ RCCs, increasing levels of GalNAc-T3 expression were not associated with worse clinicopathological features or longer survival (data not shown).
GalNAc-T3 represents an independent prognostic indicator for RCC
To assess whether GalNAc-T3/6 expressions were independent predictors of post-operative DSS, a Cox proportional-hazards model was created in a forward fashion including only covariates that had statistically significant correlations with DSS, using an inclusion threshold of P<0.05 (Table 3). Univariate analysis showed that ECOG PS, tumour size, Fuhrman’s grade, presence of VI and necrosis, and GalNAc-T3/6+ status were significant predictors of poorer survival (P<0.001, =0.002, <0.001, <0.001, =0.005,<0.001, and 0.03, respectively). Furthermore, multivariate analysis demonstrated that, after correction for confounding variables, GalNAc-T3+ expression remained an independent prognostic indicator for DSS, as did ECOG PS, Fuhrman’s grade, and VI (P=0.03, 0.02, 0.002 and 0.001, respectively).
Complementary correlations between GalNAc-T3 and -T6
Besides, this cohort was divided in two ways: (i) double negative vs either GalNAc-T3+ or -T6+ and (ii) GalNAc-T3+-only vs GalNAc-T6+-only (Figure 3A–C); the Kaplan–Meier method was used to verify them. The DSS of RCC patients showed significance and borderline significance (-T3: P<0.001 and -T6: P=0.06, respectively) in the former (i) classification approach, but not in the latter (ii) P=0.3, indicating that there were complementary, but not competitive, correlations between GalNAc-T3 and -T6.
Competitive correlations between GalNAc-T3 and adhesion molecule
Immunohistochemical expressions of β-catenin and E-cadherin were present in 144 (57%) and 65 (26%) of 254 specimens, respectively. When GalNAc-T3 and β-catenin expressions were split into groups of either positive or negative, their immunoprofiles were 10.2 –/–, 33.1, +/−, 35.4 −/+, and 21.3% +/+. These +/– and –/+ subgroups, present in 84 and 90 cases, respectively, showed significant correlation with VI (P<0.001). The DSS of RCC patients, similarly divided into two: (i) –/– vs +/– (Figure 4A) or –/+ (Figure 4B), and (ii) +/– vs –/+ (Figure 4C), revealed significant differences (P<0.01; Figure 4C) only in the latter (ii) classification approach, indicating that there were significantly competitive correlations between the GalNAc-T3 and β-catenin (Tables 2 and 3). Actually, VI components of high-grade RCCs showed immunohistochemical, GalNAc-T3+ but β-catenin– expression (Supplementary Figure 3). By contrast, no significant immunoprofile relationships were detected between GalNAc-T3 and E-cadherin (data not shown).
Discussion
In the current large cohort, we showed how GalNAc-T3 and -T6 expressions could be closely related to various clinicopathological characteristics of the 254 patients with RCCs, using antihuman polyclonal GalNAc-Ts antibodies raised against the distinctive synthetic peptides. Our collected data, including immunofluorescence or western blotting after siRNA knockdown, can confirm that those antibodies used in the present studies have not only been very sensitive to GalNAc-T3/6 but also can support that the selective staining with GalNAc-T3, as shown here in RCC samples, reflects significant upregulation of specific glycosyltransferases concomitant with specific and functional effects, but not of merely increased Golgi glycosylation in rapidly growing RCC cells. The present findings indicate, for the first time, that GalNAc-T3 is an independent novel and powerful marker for poor prognosis in patients with RCCs, whereas GalNAc-T6 is not. Our results show GalNAc-T3+ expression in RCC (1) to be significantly closely related with poor ECOG PS, large tumour size, high Fuhrman’s grade, and presence of VI and necrosis, manifesting as advanced-stage under the TNM system of UICC, that is, poorly differentiated characteristics or invasive/aggressive behaviours; (2) has significantly high co-expression with, and has potentially complementary relevance to, GalNAc-T6; and (3) apparently ameliorates adhesive effects together with significant low expression of β-catenin, seemingly leading to severe VI. A diagram depicting GalNAc-T3’s relationships from this study is summarised in Supplementary Figure 4. Furthermore, we can present a critical RCC-specific molecule, GalNAc-T3, which could potentially be a tumour marker or therapeutic target for RCC.
O-linked glycans are aberrantly expressed in many epithelial cancers with the presentation of shortened glycan structures and changes in sialylation patterns, which could affect differentiation, adhesiveness, and invasion of various cancer cells (Brockhausen, 1999; Schwientek et al, 2002; Ten Hagen et al, 2003). Our immunohistochemical examination in RCC displayed a subcellular cytoplasmic pattern for GalNAc-Ts, apparently localised to the Golgi apparatus (Grabenhorst and Conradt, 1999). In contrast, no or very weak staining was noted in adjacent normal tubular epithelium, although proximal tubule epithelial cells were rarely stained for GalNAc-T3. This implies its potentially crucial role in acquired renal carcinogenesis through aberrant O-linked glycosylation. GalNAc-T3 could be a specific diagnostic tumour marker for RCC. As GalNAc-Ts appear in external secretions such as the colostrum (Hagen et al, 1993; Bennett et al, 1996), GalNAc-T3 could be secreted into body fluids as a quantitative soluble marker. Moreover, current data suggest that GalNAc-T3 is an ideal therapeutic target with minimum risk of side effects, a nontrivial consideration, as adverse reactions caused by anticancer agents are a serious issue in clinical management. We reported that positive GalNAc-T3 expression is found in normal pancreatic ducts and bronchial epithelium (Dosaka-Akita et al, 2002; Li et al, 2011), and many carcinoma types (Shibao et al, 2002; Gu et al, 2004; Ishikawa et al, 2004; Miyahara et al, 2004; Yamamoto et al, 2004; Landers et al, 2005; Inoue et al, 2007; Li et al, 2011). Obviously, however, the utility of GalNAc-T3 inhibitors as therapeutic modalities requires much further study.
Our obtained results accord with studies of several other carcinomas, including colon (Shibao et al, 2002), gastric (Ishikawa et al, 2004), gallbladder (Miyahara et al, 2004), prostate (Landers et al, 2005), and extrahepatic bile duct (Inoue et al, 2007) epithelial cancers, where upregulated GalNAc-T3 expression was closely associated with more aggressive tumour behaviour and poor outcome. The present cohort study shows GalNAc-T3 as a powerful and independent negative indicator of DSS in patients with RCC and, by extension, GalNAc-T3 may be a novel prognostic marker for an array of different cancer types. Reportedly, the overall 5-year survival rate is 60% in RCC, but <10% in RCC patients with metastases (Cohen and McGovern, 2005). Cytokines or novel targeted therapies also seem to be of limited benefit, even after complete resection of primary RCCs (Escudier et al, 2007; Motzer et al, 2009). N-acetylgalactosaminyltransferases-3 expression patterns in primary RCC should allow improved patient selection for adjuvant systemic therapies soon after surgery and prediction of appropriate post-operative courses. Although relapse occurs in 20–30% of RCC surgical cases (Zisman et al, 2002), there have been no reliable predictors of progressive potential to date (Chow et al, 1999; Cohen and McGovern, 2005). N-acetylgalactosaminyltransferases-3, used as a molecular marker, may be able to refine individual risk stratification and treatment plans, possibly including specific anti-GalNAc-T3 enzymes. This intriguing prospect warrants more experimentation on the potential invasive and metastatic functions of GalNAc-T3 in RCC. Univariate and multivariate Cox survival analyses confirm that GalNAc-T3 expression is an independent prognostic factor in RCC if the cut-off value is set at 10%, but not at 30 or 80%. There seems to be some danger of data fitting in arbitrary cut-off values; however, the blinded protocol design used should prevent that it would have influenced the final conclusive results. Nevertheless, this discrepancy could be due to the heterogeneity of cancer or the size of cohort, and further experiments are thus needed to address the methodology standardisation for GalNAc-T3 in clinical specimens after collecting and investigating a much larger number of surgical RCC cases.
The GalNAc-T3 exhibits a high sequence homology to the GalNAc-T6 gene, located on chromosome 12q13 and containing 10 exons of 1869 base pairs that encode a type-II transmembrane protein (Bennett et al, 1999). As GalNAc-T3 and -T6 are thus nearly identical, but distinct, substrate specificities and represent the only two enzymes to form glycosylated oncofoetal fibronectin in vitro (Wandall et al, 1997; Bennett et al, 1998), biosynthesis of the glycosylated form of oncofoetal fibronectin in vivo quite possibly results from cooperating expression of an alternative spliced variant of fibronectin containing the glycosylation site, initiated by at least either of two GalNAc-T3 or -T6 (Wandall et al, 2007). More recently, our laboratory also found GalNAc-T3 and -T6 to co-express significantly in a cohort of surgical pancreatic cancer cases, although they might function separately (Li et al, 2011). Although similar to our obtained data regarding GalNAc-T3, the present findings on GalNAc-T6 are also consistent with our own and others’ previous studies in part, showing de novo expression of GalNAc-T6 in several other carcinomas, including oral (Wandall et al, 2007), breast (Berois et al, 2006; Freire et al, 2006), gastric (Gomes et al, 2009), and pancreas (Li et al, 2011) epithelial cancers, where the malignant cells specifically demonstrated GalNAc-T6 expression associated occasionally with more progressive tumour characteristics and bad prognosis. On the basis of these features, we can easily hypothesise that GalNAc-T3 in vivo should be concurrently expressed with GalNAc-T6 in RCC as well. Here, we have revealed a significantly close relationship between these two GalNAc-Ts, and survival analyses further show significant differences, especially in a double-negative vs either-positive classification approach, which suggests complementary correlations between GalNAc-T3 and -T6. In this context, upregulation of GalNAc-T3 within the malignant transformation, differentiation, invasion, or progression of RCC cells could partly depend on that of GalNAc-T6 in both synergistic and compensatory ways. However, this relationship remains to be elucidated more in detail; further follow-up cohort and molecular studies are needed to determine the comprehensive mechanisms between GalNAc-T3 and -T6 expressions.
To further clarify GalNAc-T3’s function in RCC, we examined expression patterns of two cell-adhesion molecules, β-catenin and E-cadherin, which critically affect cell morphology and carcinogenesis (Yuan et al, 2007; Park et al, 2010). A study that focused on modulation of cell-adhesion function by GalNAc-Ts suggested that GalNAc-T6 is involved in mammary carcinogenesis through aberrant glycosylation and stabilisation of an oncoprotein and O-glycoprotein mucin 1 (MUC1), accompanied by the downregulation of E-cadherin and β-catenin (Park et al, 2010). Correspondingly, the GalNAc-T3+ expression in RCC was also found to suppress one of the two cell-adhesion molecular markers, β-catenin, probably resulting in anti-adhesive effects and leading to vessel permeation, that is, severe VI and advanced TNM stage, as shown here. Furthermore, our preliminary findings suggest that the protein glycosylated by GalNAc-T3 in RCC is MUC1; it is partly supported by the indication that MUC1 could be a therapeutic agent against RCC (Aubert et al, 2009). We could also hypothesise that GalNAc-T3 promotes RCC invasion and metastasis by affecting cell–stromal interaction and cell–cell adhesion. A recent paper has confirmed that GalNAc-T6 also accelerates epithelial–mesenchymal transition and facilitates metastases in breast cancer (Park et al, 2011). Although further in-depth analyses are needed to elucidate this field, our original data both indicate the crucial role of aberrant O-linked glycosylation via GalNAc-T3 in renal carcinogenesis and RCC invasiveness, but also imply the potential of GalNAc-T3 as a target in RCC treatment. It might be suggested that a gene therapy with RNA interference for GalNAc-T3 is very promising.
In conclusion, our findings show that GalNAc-T3+ expression in RCC is significantly related to histopathological phenotypes with poor prognoses, for example, those with VI, high Fuhrman’s grade, or advanced TNM stage. Moreover, outcomes of patients whose RCCs had GalNAc-T3+ were markedly worse compared with those with GalNAc-T3− RCCs. These analyses indicate, for the first time, that GalNAc-T3 is a novel and useful independent predictor of histologically high-grade tumour and poor prognosis in patients with RCC.
References
Aubert S, Fauquette V, Hémon B, Lepoivre R, Briez N, Bernard D, Van Seuningen I, Leroy X, Perrais M (2009) MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res 69 (14): 5707–5715.
Baldewijns MM, van Vlodrop IJ, Schouten LJ, Soetekouw PM, de Bruïne AP, van Engeland M (2008) Genetics and epigenetics of renal cell cancer. Biochim Biophys Acta 1785 (2): 133–155.
Bennett EP, Hassan H, Clausen H (1996) cDNA cloning and expression of a novel human UDP-N-acetyl-alpha-D-galactosamine. Polypeptide N-acetylgalactosaminyltransferase GalNAc-t3. J Biol Chem 271 (29): 17006–17012.
Bennett EP, Hassan H, Mandel U, Hollingsworth MA, Akisawa N, Ikematsu Y, Merkx G, van Kessel AG, Olofsson S, Clausen H (1999) Cloning and characterization of a close homologue of human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase-T3, designated GalNAc-T6. Evidence for genetic but not functional redundancy. J Biol Chem 274 (36): 25362–25370.
Bennett EP, Hassan H, Mandel U, Mirgorodskaya E, Roepstorff P, Burchell J, Taylor-Papadimitriou J, Hollingsworth MA, Merkx G, van Kessel AG, Eiberg H, Steffensen R, Clausen H (1998) Cloning of a human UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase that complements other GalNAc-transferases in complete O-glycosylation of the MUC1 tandem repeat. J Biol Chem 273 (46): 30472–30481.
Berois N, Mazal D, Ubillos L, Trajtenberg F, Nicolas A, Sastre-Garau X, Magdelenat H, Osinaga E (2006) UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 as a new immunohistochemical breast cancer marker. J Histochem Cytochem 54 (3): 317–328.
Brockhausen I (1999) Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta 1473 (1): 67–95.
Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr (1999) Rising incidence of renal cell cancer in the United States. Jama 281 (17): 1628–1631.
Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353 (23): 2477–2490.
Dosaka-Akita H, Kinoshita I, Yamazaki K, Izumi H, Itoh T, Katoh H, Nishimura M, Matsuo K, Yamada Y, Kohno K (2002) N-acetylgalactosaminyl transferase-3 is a potential new marker for non-small cell lung cancers. Br J Cancer 87 (7): 751–755.
Dwek RA (1996) Glycobiology: toward understanding the function of sugars. Chem Rev 96 (2): 683–720.
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370 (9605): 2103–2111.
Finley DS, Pantuck AJ, Belldegrun AS (2011) Tumor biology and prognostic factors in renal cell carcinoma. Oncologist 16 (Suppl 2): 4–13.
Freire T, Berois N, Sonora C, Varangot M, Barrios E, Osinaga E (2006) UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int J Cancer 119 (6): 1383–1388.
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6 (7): 655–663.
Gomes J, Marcos NT, Berois N, Osinaga E, Magalhaes A, Pinto-de-Sousa J, Almeida R, Gartner F, Reis CA (2009) Expression of UDP-N-acetyl-D-galactosamine: polypeptide N-acetylgalactosaminyltransferase-6 in gastric mucosa, intestinal metaplasia, and gastric carcinoma. J Histochem Cytochem 57 (1): 79–86.
Grabenhorst E, Conradt HS (1999) The cytoplasmic, transmembrane, and stem regions of glycosyltransferases specify their in vivo functional sublocalization and stability in the Golgi. J Biol Chem 274 (51): 36107–36116.
Gu C, Oyama T, Osaki T, Li J, Takenoyama M, Izumi H, Sugio K, Kohno K, Yasumoto K (2004) Low expression of polypeptide GalNAc N-acetylgalactosaminyl transferase-3 in lung adenocarcinoma: impact on poor prognosis and early recurrence. Br J Cancer 90 (2): 436–442.
Hagen FK, Van Wuyckhuyse B, Tabak LA (1993) Purification, cloning, and expression of a bovine UDP-GalNAc: polypeptide N-acetyl-galactosaminyltransferase. J Biol Chem 268 (25): 18960–18965.
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4 (1): 45–60.
Igarashi T, Izumi H, Uchiumi T, Nishio K, Arao T, Tanabe M, Uramoto H, Sugio K, Yasumoto K, Sasaguri Y, Wang KY, Otsuji Y, Kohno K (2007) Clock and ATF4 transcription system regulates drug resistance in human cancer cell lines. Oncogene 26 (33): 4749–4760.
Inoue T, Eguchi T, Oda Y, Nishiyama K, Fujii K, Izumi H, Kohno K, Yamaguchi K, Tanaka M, Tsuneyoshi M (2007) Expression of GalNAc-T3 and its relationships with clinicopathological factors in 61 extrahepatic bile duct carcinomas analyzed using stepwise sections -special reference to its association with lymph node metastases. Mod Pathol 20 (2): 267–276.
Ishikawa M, Kitayama J, Nariko H, Kohno K, Nagawa H (2004) The expression pattern of UDP-N-acetyl-alpha-d-galactosamine: polypeptide N-acetylgalactosaminyl transferase-3 in early gastric carcinoma. J Surg Oncol 86 (1): 28–33.
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48 (3): 452–458.
Landers KA, Burger MJ, Tebay MA, Purdie DM, Scells B, Samaratunga H, Lavin MF, Gardiner RA (2005) Use of multiple biomarkers for a molecular diagnosis of prostate cancer. Int J Cancer 114 (6): 950–956.
Li Z, Yamada S, Inenaga S, Imamura T, Wu Y, Wang KY, Shimajiri S, Nakano R, Izumi H, Kohno K, Sasaguri Y (2011) Polypeptide N-acetylgalactosaminyltransferase 6 expression in pancreatic cancer is an independent prognostic factor indicating better overall survival. Br J Cancer 104 (12): 1882–1889.
Miyahara N, Shoda J, Kawamoto T, Furukawa M, Ueda T, Todoroki T, Tanaka N, Matsuo K, Yamada Y, Kohno K, Irimura T (2004) Expression of UDP-N-acetyl-alpha-D-galactosamine-polypeptide N-acetylgalactosaminyltransferase isozyme 3 in the subserosal layer correlates with postsurgical survival of pathological tumor stage 2 carcinoma of the gallbladder. Clin Cancer Res 10 (6): 2090–2099.
Miyamoto N, Izumi H, Noguchi T, Nakajima Y, Ohmiya Y, Shiota M, Kidani A, Tawara A, Kohno K (2008) Tip60 is regulated by circadian transcription factor clock and is involved in cisplatin resistance. J Biol Chem 283 (26): 18218–18226.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27 (22): 3584–3590.
Naito S, Kanamori T, Hisano S, Tanaka K, Momose S, Kamata N (1982) Human renal cell carcinoma: establishment and characterization of two new cell lines. J Urol 128 (5): 117–1121.
Nomoto M, Izumi H, Ise T, Kato K, Takano H, Nagatani G, Shibao K, Ohta R, Imamura T, Kuwano M, Matsuo K, Yamada Y, Itoh H, Kohno K (1999) Structural basis for the regulation of UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyl transferase-3 gene expression in adenocarcinoma cells. Cancer Res 59 (24): 6214–6222.
Park JH, Katagiri T, Chung S, Kijima K, Nakamura Y (2011) Polypeptide N-acetylgalactosaminyltransferase 6 disrupts mammary acinar morphogenesis through O-glycosylation of fibronectin. Neoplasia 13 (4): 320–326.
Park JH, Nishidate T, Kijima K, Ohashi T, Takegawa K, Fujikane T, Hirata K, Nakamura Y, Katagiri T (2010) Critical roles of mucin 1 glycosylation by transactivated polypeptide N-acetylgalactosaminyltransferase 6 in mammary carcinogenesis. Cancer Res 70 (7): 2759–2769.
Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA (2001) Glycosylation and the immune system. Science 291 (5512): 2370–2376.
Schwientek T, Bennett EP, Flores C, Thacker J, Hollmann M, Reis CA, Behrens J, Mandel U, Keck B, Schäfer MA, Haselmann K, Zubarev R, Roepstorff P, Burchell JM, Taylor-Papadimitriou J, Hollingsworth MA, Clausen H (2002) Functional conservation of subfamilies of putative UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosaminyltransferases in Drosophila, Caenorhabditis elegans and mammals. J Biol Chem 277 (25): 22623–22638.
Shibao K, Izumi H, Nakayama Y, Ohta R, Nagata N, Nomoto M, Matsuo K, Yamada Y, Kitazato K, Itoh H, Kohno K (2002) Expressions of UDP-N-Acetyl-α-D-galactosaminepolypeptide GalNAc N-acetylgalactosaminyl transferase-3 in relation to differentiation and prognosis in patients with colorectal carcinoma. Cancer 94 (7): 1939–1946.
Siegel R, Naishadham D, Jemal A (2012) Cancer Statistics 2012. CA Cancer J Clin 62 (1): 10–29.
Sobin LH, Gospodarowicz MK, Wittekind Ch (2009) TNM Classification of Malignant Tumours 7th edn Wiley-Blackwell: Hoboken.
Ten Hagen KG, Fritz TA, Tabak LA (2003) All in the family: the UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 13 (1): 1R–16R.
Wandall HH, Dabelsteen S, Sorensen JA, Krogdahl A, Mandel U, Dadelsteen E (2007) Molecular basis for the presence of glycosylated onco-foetal fibronectin in oral carcinomas: the production of glycosylated onco-foetal fibronectin by carcinoma cells. Oral Oncol 43 (3): 301–309.
Wandall HH, Hassan H, Mirgorodskaya E, Kristensen AK, Roepstorff P, Bennett EP, Nielsen PA, Hollingsworth MA, Burchell J, Taylor-Papadimitriou J, Clausen H (1997) Substrate specificities of three members of the human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferases family, GalNAc-T1, -T2, and -T3. J Biol Chem 272 (38): 23503–23514.
Yamamoto S, Nakamori S, Tsujie M, Takahashi Y, Nagano H, Dono K, Umeshita K, Sakon M, Tomita Y, Hoshida Y, Aozasa K, Kohno K, Monden M (2004) Expression of uridine diphosphate N-acetyl-alpha-D-galactosamine: polypeptide N-acetylgalactosaminyl transferase 3 in adenocarcinoma of the pancreas. Pathobiology 71 (1): 12–18.
Yuan Z, Wong S, Borrelli A, Chung MA (2007) Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun 362 (3): 740–746.
Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, deKernion JB, Figlin RA, Belldegrun AS (2002) Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol 20 (23): 4559–4566.
Acknowledgements
We thank Dr and Professor Akihide Tanimoto, Department of Molecular and Cellular Pathology, Kagoshima University Graduate School of Medical and Dental Sciences, for excellent technical assistance and helpful comments, and Hiroko Isagai, Hana Nishimura, and Naoko Une for their expert technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research (24790394, 20590416, and 19590413) from the Ministry of Education, Culture, Sports, Science, and Technology, Tokyo, Japan (to SY, KK, and YS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kitada, S., Yamada, S., Kuma, A. et al. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br J Cancer 109, 472–481 (2013). https://doi.org/10.1038/bjc.2013.331
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.331
Keywords
This article is cited by
-
Global view of human protein glycosylation pathways and functions
Nature Reviews Molecular Cell Biology (2020)
-
N-Acetylglucosaminyltransferase III (GnT-III) but not N-Acetylgalactosaminyltransferase-6 and 8 are Differentially Expressed in Invasive and In Situ Ductal Carcinoma of the Breast
Pathology & Oncology Research (2019)
-
Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression
Journal of Hematology & Oncology (2018)
-
Genome-wide analysis of endogenously expressed ZEB2 binding sites reveals inverse correlations between ZEB2 and GalNAc-transferase GALNT3 in human tumors
Cellular Oncology (2018)
-
CD163-positive cancer cells are potentially associated with high malignant potential in clear cell renal cell carcinoma
Medical Molecular Morphology (2018)