Abstract
Background:
To assess the clinical impact of the two histological types as designated in the proposed model for ovarian tumourigenesis in primary epithelial ovarian, fallopian tube or peritoneal cancer (EOC) patients.
Methods:
All consecutive EOC patients (n=632) after primary tumour debulking in our institution (09/2000–08/2010) were classified into one of two groups: type I tumours (n=100; 15.8%) composed of low-grade serous, low-grade endometrioid, clear cell, mucinous and transitional carcinomas; and Type II tumours (n=532; 84.1%) composed of high-grade serous, high-grade endometrioid, undifferentiated and malignant mixed-mesodermal tumours. Kaplan–Meier and logistic/Cox-regression analyses were performed to assess the impact of histological type on surgical outcome and survival.
Results:
Type II patients had a significantly higher incidence of advanced disease (FIGO III/IV) than Type I patients (79.8% vs 38%, respectively; P<0.001). Median CA125 values (438 vs 93 U ml−1; P=0.001); operative time (258 vs 237 min; P=0.001); and incidence of incomplete tumour resection (34.4% vs 15%; P<0.001) were significantly higher in patients with Type II. During a mean follow-up time of 23 months (range: 1–106), 17% of patients with type I vs 34.8% of patients with type II tumours relapsed and/or died (P<0.001). Overall survival (P=0.021) and progression-free survival (P=0.003) were also significantly higher in patients with type I tumours. Multivariate analysis, while identifying postoperative tumour residuals, positive lymph nodes and extrapelvic dissemination as independent predictors of survival, failed to demonstrate any prognostic significance of histological type.
Conclusion:
Type I EOC patients appear to present at earlier stages have significantly higher survival and more optimal surgical outcome compared with type II patients. However, in advanced stages, histology loses significance as an independent prognosticator.
Similar content being viewed by others
Main
In the beginning of this century a novel tumour progression and origination model for ovarian carcinoma was proposed based on morphological and molecular genetic analyses. In this model, epithelial ovarian tumours are divided into two categories designated type I and type II, which correspond to the two main pathways of tumourigenesis (Singer et al, 2002; Shih and Kurman, 2004). Differences between the two types of tumours are based on clinical behaviour and evolution, genetic stability and molecular genetic profiles. As first suggested by Shih and Kurman (2004), type I tumours generally behave in an indolent manner, are genetically stable without the classic mutations such as of TP53 and tend to be confined to the ovary. On the contrary, type II tumours are characterised by a more ‘aggressive’ behaviour, the vast majority display TP53 mutations and are distinguished by rapid evolution (Shih and Kurman, 2005; Kurman and Shih, 2008, 2010). As a result of this hypothesis a 2-tier, as opposed to the 3-tier, system has been proposed, in which tumours would be subdivided into low-grade and high-grade differentiation. This approach is supposed to be more simplified, reproducible, and apparently closer to the novel biological evidence of epithelial ovarian, fallopian tube or peritoneal cancer (EOC) pathway development (Shih and Kurman, 2004; Bell, 2005; Kurman et al, 2008; Vang et al, 2009).
Even though various histopathological analyses and evaluations have been conducted to attempt to define the clinicopathological and biological features of these two tumour classifications, no analysis has yet been presented that assesses these two tumour ‘types’ relative to actual clinical outcome in a large population-based study. The present work is an attempt to assess and identify the actual clinical impact on both surgical outcome and survival of these two histological types in a large cohort of primary EOC patients who underwent optimal primary tumour debulking and first-line platinum-based chemotherapy.
Materials and Methods
A systematic retrospective analysis of a prospectively maintained database was performed to evaluate the intraoperative tumour dissemination pattern, surgical outcome and survival of all women operated on for primary EOC in the European Competence Center for Ovarian Cancer at the Charité University of Berlin between September 2000 and August 2010. All patients who underwent a neo-adjuvant chemotherapy approach with subsequent interval debulking surgery were excluded from the present analysis (n=53). Patients were classified into two groups: type I and type II as introduced by Shih and Kurman in the proposed model for ovarian tumourigenesis (Shih and Kurman, 2004, 2005). Type I included all low-grade serous papillary and low-grade endometrioid, all mucinous, clear cell and transitional cell cancers. Type II included all high-grade serous papillary and high-grade endometrioid ovarian cancers, all mixed histologies and all carcinosarcomas. A total of 632 evaluable patients were included; 100 type I (15.8%) and 532 type II (84.17%).
The histopathological assessment was performed prospectively at the Institute of the Charité, all tumours were assessed by two pathologists. To control for tumour heterogeneity multiple large H&E sections of the primary ovarian lesion, lymph nodes and all intraperitoneal lesions were examined. Histological tumour typing was performed according to the WHO guidelines. Grading was performed according to Silverberg. Histological results were retrospectively regrouped as indicated by Shih and Kurman (2004). In order to detect possible misclassification, we re-examined the type I tumours with advanced FIGO stages IIIc or IV (n=33) and confirmed histological diagnosis.
All operations were performed by one of three gynaecologic oncologic surgeons. Staging was performed and defined in accordance with the FIGO criteria for ovarian cancer (FIGO – International Federation of Gynecology and Obstetrics, 1987). Each primary surgery was performed per midline laparotomy aiming at maximal tumour reduction and adequate staging. A summary of the surgical procedures performed is presented in detail in Table 2.
In every patient, a detailed tumour pattern was intraoperatively assessed based on the surgical procedures performed and through a systematic interview of the surgical team. Postoperatively all histological findings and associated data were entered into a validated documentation system (IMO: Intraoperative-Mapping-of-Ovarian-Cancer), specifically developed for ovarian neoplasms with particular focus on the description of the tumour pattern, maximal tumour burden, postoperative tumour residuals (0, <0.5, <1, <2, >2 cm) and the amount of preoperative ascites (none, < or >500 ml). IMO represents a detailed surgical and histopathological documentation system developed in our clinic in order to obtain a better and more objective description of the ovarian tumour spread within the abdominal cavity and to define more precisely the histopathological features of the malignancy (Sehouli et al, 2003, 2009, 2010a, 2010b; Fotopoulou et al, 2010, 2011). Within the Tumour Bank Ovarian Cancer project (www.toc.network.de), tumour tissue, ascites, serum and blood were collected from each EOC patient. The patients’ informed consent was always obtained prior to surgery and sample collection and documentation. The levels and fields according to which the abdominal cavity is divided into are presented in Figure 1.
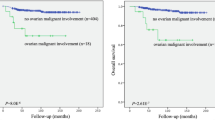
Tumour dissemination patterns in type I vs type II primary ovarian cancer (OC), according to the ‘Intraoperative Mapping of Ovarian Cancer’ documentation tool and survival curves according to histology depicted separately for FIGO (International Federation of Gynecology and Obstetrics) stages I/II and III/IV.
Follow up
All relevant patient data, including medical history, follow-up and survival data, were abstracted from the patients’ records. Survival data were last updated on 02/2011 based on the patient's files and/or responses from their physicians or insurance companies.
Patients were regularly evaluated post-treatment for evidence of disease recurrence. Clinical examinations, transvaginal and transabdominal ultrasound, serum CA125 (if preoperative value was elevated) assays were performed every 3 months. A CT/MRI scan was ordered if the above examinations revealed any pathology. Recurrence was defined by pathological, clinical, radiological or sonographical findings. Time to recurrence was defined as the date of radiological evidence of recurrence and not merely due to an isolated increase of CA125.
Statistics
Fisher's exact test, Kendall's tau-b and Mann–Whitney's U-test were used for univariate analysis, where appropriate. Crude and adjusted odds ratios with corresponding 95% confidence intervals (95% CI) were obtained using logistic regression analysis. Estimates of survival were calculated using the Kaplan–Meier method, and log-rank tests were used for univariate statistical comparisons. The relative value of individual variables as independent predictors of overall survival (OS) and progression-free survival (PFS) was analysed with the multivariate Cox proportional hazard-regression model. Adjusted hazard ratios (HRs) and 95% CI for prognostic factors were estimated. All data were analysed using PASW Statistics 18 (SPSS, Chicago, IL, USA), and P<0.05 (two-tailed) was considered statistically significant. The follow-up time was calculated starting on the day of surgery.
Results
A total of 632 patients were included in the present analysis. One hundred patients (15.8%) were classified as type I, whereas 532 patients (84.1%) were of type II. The distribution of low-grade serous, clear cell and mucinous carcinomas by FIGO stage was as follows: low-grade serous FIGO I: 4 (17.4%), FIGO II: 2 (8.7%), FIGO III: 17 (74%) and FIGO IV: 0; clear cell FIGO I: 8 (66.7%), FIGO II: 2 (16.7%), FIGO III: 2 (16.7%) and FIGO IV: 0; mucinous FIGO I: 19 (56%), FIGO II: 1 (2.9%), FIGO III: 8 (23.5%) and FIGO IV: 17.6% (P<0.001, by χ2-test). The detailed distribution of the 33 advanced type I tumours (i.e., IIIc/IV) was as follows: low-grade serous 13 (39.4%), mucinous 14 (42.4%), clear cell 2 (6.1%) and transitional cell 4 (12.1%). Patient characteristics are presented in detail in Table 1. Type I patients were significantly younger, presented at significantly earlier FIGO stages and had lower rates of positive lymph nodes, preoperative ascites and CA125 values.
During a mean follow-up time of 23 months (range: 1–106; median: 15), 202 patients (32%) relapsed and died. Estimated 5-year OS rates were 56.3% (95% CI: 38.3–84.0%) for type I patients vs 39.3% (95% CI: 32.6–46.0%) for type II patients (P=0.021) and thus statistically significantly different. Estimated 2-year PFS rates were also significantly better for type I compared with type II patients: 59.8% (95% CI: 46.1–73.4%) vs 44.9% (95% CI: 39.6–50.2%); P=0.003. Survival curves are presented in Figure 1. However, when considering only advanced FIGO IIIc/IV patients, both OS and PFS were not statistically significantly different between the two groups: median OS was 35 months (95% CI: 13.06–56.93) for type I vs 40 months (95% CI: 33.5–46.47) for type II patients (P=0.779) and median PFS was 20 months (95% CI: 0.000–42.38) for type I vs 17 months (95% CI: 14.49–19.5) for type II patients (P=0.714).
Type I patients demonstrated higher rates of platinum sensitivity after first-line platinum-based chemotherapy (85.4% vs 77.5%) relative to type II patients, but failed to reach any statistical significance (P=0.314).
Detailed surgical procedures performed during primary tumour debulking are presented in Table 2. Type I patients underwent significantly less frequent para aortic lymph node dissection, large bowel resection, an extensive peritonectomy and a diaphragmatic resection, and had a significantly lower overall complication rate during a significantly less median operative time. These differences lost statistical significance when comparing only the FIGO stages III and IV patients. Of the 33 type I patients with advanced disease, the vast majority (84%) underwent an optimal tumour debulking; i.e., 72% were surgically completely tumour-free and 12% had tumour residuals of less than 0.5 cm. These rates were equivalent to the optimal tumour resection rates of type I patients across all stages.
Tumour dissemination rates were significantly lower in all three abdominal levels (i.e., IMO 1–3) in type I patients. Exact values are presented in Figure 1. In comparison with type II patients, type I patients had lower rates of tumour involvement of the omentum (33% vs 66%; P<0.001), the Pouch of Douglas (11% vs 23%; P=0.007), the pelvic peritoneum (17% vs 30%; P=0.007), the diaphragm (19% vs 42%; P<0.001), the serosa of the small (15% vs 31%; P=0.001) and large (24% vs 51%; P<0.001) intestine, the mesentery (20% vs 41%; P<0.001), as well as significantly lower rates of diffuse peritoneal carcinosis (42% vs 78%; P<0.001). Although the mean number of extracted lymph nodes was not significantly different between the two patient groups (28 for type I vs 31 for type II; P=0.25), the mean number of affected lymph nodes was significantly higher in type II patients (5.8 vs 1.5; P=0.001).
The mean number of IMO fields with tumour residuals was significantly higher in type II vs type I patients (0.45 vs 0.87; P<0.001); the number of IMO fields with maximal tumour load was also significantly greater in type II patients (mean: 1.1 vs 1.4; P<0.001), as was the total number of IMO fields affected with tumour (mean: 2.7 vs 4.3; P<0.001).
On multivariate analysis, comparing the different histological entities stage by stage, only a positive lymph node status was identified as independent predictor negatively affecting OS in early tumour stages 1 and 2, while absence of ascites had a significant protective effect. For the more advanced tumour stages 3 and 4, positive lymph node status as well as any postoperative tumour residuals and mucinous histology appeared to negatively affect survival. These data are presented in Table 3.
Interestingly, when evaluating only the tumour-free-operated patients, high-grade serous cancers (HR: 3.83; 95% CI: 1.062–13.8; P=0.04), all the other type II histologies (HR: 4.89; 95% CI: 1.18–20.14; P=0.03) and multifocal tumour dissemination (HR: 1.8; 95% CI: 1.08–2.9; P=0.023) were the only independent predictors negatively affecting survival. Ascites, lymph node status and age did not seem to have any significant effect.
Independent predictors of tumour recurrence were for the early stages 1 and 2, a positive lymph node status, while absence of ascites appeared to have a significant protective effect. For the more advanced tumour stages 3 and 4, a positive lymph node status and the presence of tumour residuals were of prognostic significance for relapse. Data are presented in Table 3.
Predictors of complete tumour resection and risk factors for extrapelvic tumour dissemination are presented in Tables 4 and 5.
Regarding operative morbidity, only intestinal resection was shown to be an independent risk factor, between the histological types, advanced age, ascites, FIGO stage, lymph node dissection, operative time and tumour residuals.
Discussion
In the present analysis we evaluated the impact of histological type, as defined by the dualistic model of carcinogenesis, on surgical and clinical outcome after primary tumour debulking of EOC patients. We demonstrated that type I patients were significantly younger at initial presentation of disease compared with patients with type II tumours. Moreover, in type I patients the disease presented at significantly earlier stages, with lower incidence of ascites and lymph node involvement, significantly higher rates of optimal tumour debulking and, consequently, significantly better overall and PFS rates. Nevertheless, when considering only the subgroup of patients with advanced FIGO stages III and IV, the histological type did not retain any significant prognostic value on survival nor on surgical outcome. Furthermore, we could not identify any significant independent effect of the different histological entities on tumour respectability or operative morbidity. In advanced stages of disease, only mucinous histology had a negative impact on survival when compared with low-grade serous tumours, whereas high-grade serous histology was identified as a significant risk factor for extrapelvic tumour dissemination.
Interestingly, in the tumour-free-operated patients, type II cancers were together with a multifocal tumour dissemination, the only significant risk factor negatively affecting OS.
This is, to our knowledge, the first report of assessing the impact and significance of tumour histology using a validated and systematic documentation tool such as IMO, where intraoperative tumour dissemination, operative procedures and tumour residuals are described and recorded in a prospective and systematic manner using specifically designed schemes and figures (see Figure 1), avoiding potential bias and errors in the assessment of tumour dissemination and site of tumour residuals. In previous studies, Gershenson et al (2009) investigated the chemoresistance of recurrent low-grade serous ovarian carcinoma compared with high-grade ovarian cancers. The authors questioned whether the latter's high rate of stable disease was due more to the tumour's biology or to the influence of chemotherapy.
Even though the existing guidelines regarding EOC do not officially include the histological subtype in the decision-making process, there is increasing evidence that indicates histology has a significant role in the overall patients’ outcome and prognosis (Goff et al, 1996; Ho et al, 2004; Pectasides et al, 2005; Bamias et al, 2010; Wimberger et al, 2010; Zaino et al, 2010), while multiple papers describe treating low-grade serous, mucinous and clear cell cancers differently. The Gynecologic Oncology Group (GOG) has designed several large multicentre phase III trials, specifically for low-grade serous cancers alone, mucinous alone and clear cell alone, attempting to treat, for instance, mucinous type tumours similar to intestinal cancers with Oxaliplatin and Capecitabine ± Bevacizumab.
Epithelial ovarian, fallopian tube or peritoneal cancer is complex at both the histopathological and molecular level, being composed of several histological subtypes that are, in of themselves, heterogeneous and contain distinct molecular signatures (Blagden and Gabra, 2009). It appears that rare histological subtypes, including mucinous or clear cell adenocarcinomas, respond rather poorly to the standard first-line chemotherapy combination of carboplatin and paclitaxel, and are associated with a more dismal outcome compared with their serous counterparts (Blagden and Gabra, 2008). This issue was also addressed in a previous analysis by Goff et al (1996), where 70% of the patients with clear cell histology developed progressive disease, compared with only 29% of those with serous histology. Similar lower response rates to first-line platinum-based chemotherapy were also shown for patients with mucinous epithelial ovarian cancer (Pectasides et al, 2005; Bamias et al, 2010). In a recent retrospective analysis of the German Arbeitsgemeinschaft Gynaekologische Onkologie on a large cohort of EOC patients (Wimberger et al, 2010), multivariable analysis for OS identified mucinous histological type next to postoperative residual tumour, multiple sites of metastases and ECOG performance status as statistically significant prognostic variables.
In a further study by the GOG (Zaino et al, 2010), advanced stage mucinous adenocarcinoma of the ovary is being characterised as highly lethal with highly significantly lower OS rates compared with women with serous carcinoma (14 vs 42 months; P<0.001).
Interestingly, according to the ovarian tumourigenesis hypothesis, both clear cell and mucinous adenocarcinomas belong to the prognostically more favourable type II group. In our analysis we demonstrated that both entities are indeed associated with a more favourable prognosis, mainly due, however, to the fact that they initially present at earlier stages. In advanced stages of the disease, histological type I vs type II cancers did not show significant differences with respect to survival or surgical outcome, and advanced type I cancers behaved as aggressively as type II cancers, with mucinous histology even being associated with a significantly worse outcome compared with the serous type. How might we explain this? One theory is that it is a reflection of the p53 status. It is known that mutations in p53 are common in high-grade serous carcinomas in contrast to their low-grade serous counterparts in which mutations in p53 are rather rare. Many studies have shown that 50–80% of advanced stage disease harbours mutant p53, presumably because they might possibly originate from high-grade serous carcinomas (Shih and Kurman, 2004). In an immunohistochemical evaluation by Eltabbakha et al (2004) aiming to investigate the clinical and molecular factors associated with cytoreduction among women with advanced stage epithelial EOC, it was found that p53 expression was a highly significant predictor for cytoreducibility. Women whose tumours showed mild or moderate p53 expression were 5.6 times more likely to achieve complete cytoreduction compared with women whose tumours showed strong p53 expression (Eltabbakha et al, 2004). Projecting this to the well-established fact that in advanced stages, tumour residual disease is the most significant prognostic factor for survival and that optimal tumour debulking to microscopic residuals is regarded as the cornerstone of therapeutic management in EOC, one could surmise that p53 status has a significant impact on overall prognosis even in advanced stage disease. In our analysis we did not perform any p53 mutation analysis or an assessment of the Ras/Raf wild type, which should be noted as shortcoming of this study. We however, performed a subanalysis of the prognostically more favourable cohort of the tumour-free-operated patients and showed that type II histology was a negative prognosticator of survival. This might be hence a sign of a potential ‘higher aggressiveness’ of type II cancers after all, whereas the exact underlying mechanisms have to be investigated in future trials.
A further weakness of the present analysis is the relatively short follow-up of an average of 23 months.
It is known that underlying the general high mortality of EOC is the molecular behaviour of the disease, with ∼75% of patients presenting at an advanced clinical stage, in terms of a high-volume disease with dissemination in the entire abdominal cavity (Kurman et al, 2008; Blagden and Gabra, 2009). Type I tumours have been described as slow growing, as evidenced by the observation that they are large and often confined to the ovary at diagnosis (Shih and Kurman, 2004). This observation was corroborated in this study, where type I tumours initially presented in approximately half of the cases at an early stage restricted to the pelvis (IMO level 1), as opposed to only 14% of type II patients. This may imply that despite the fact that rare entities such as mucinous and clear cell carcinomas show a poorer response to conventional chemotherapeutic regimes, their tendency to be diagnosed at earlier stages, confined to the pelvis, may actually result in a more favourable prognosis and more beneficial profile of the type I tumours.
However, these hypotheses have to be verified in prospective morphological and molecular genetic studies, where a molecular biological profiling of the tumour will be conducted at inception of the disease and, subsequently, correlated with surgical outcome and survival. In this way a new rationale in the approach to detection, therapeutic management and follow-up may be developed, which would be closer to the actual tumour biology profiling and large-scale heterogeneity of the disease (Gershenson, 2010) and may allow a more individualised and potentially more effective management.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bamias A, Psaltopoulou T, Sotiropoulou M, Haidopoulos D, Lianos E, Bournakis E, Papadimitriou C, Rodolakis A, Vlahos G, Dimopoulos MA (2010) Mucinous but not clear cell histology is associated with inferior survival in patients with advanced stage ovarian carcinoma treated with platinum-paclitaxel chemotherapy. Cancer 116 (6): 1462–1468
Bell DA (2005) Origins and molecular pathology of ovarian cancer. Mod Pathol 18 (Suppl 2): 19–32
Blagden S, Gabra H (2008) Future directions in the management of epithelial ovarian cancer. Future Oncol 4: 403–411
Blagden S, Gabra H (2009) Promising molecular targets in ovarian cancer. Curr Opin Oncol 21 (5): 412–419
Eltabbakha GH, Mountb SL, Beattyb B, Simmons-Arnoldb L, Cooperb K, Morgan A (2004) Factors associated with cytoreducibility among women with ovarian carcinoma. Gynecol Oncol 95: 377–383
Fotopoulou C, Richter R, Braicu EI, Schmidt SC, Lichtenegger W, Sehouli J (2010) Can complete tumour resection be predicted in advanced primary epithelial ovarian cancer? A systematic evaluation of 360 consecutive patients. Eur J Surg Oncol 36 (12): 1202–1210
Fotopoulou C, Richter R, Braicu IE, Schmidt SC, Neuhaus P, Lichtenegger W, Sehouli J (2011) Clinical outcome of tertiary surgical cytoreduction in patients with recurrent epithelial ovarian cancer. Ann Surg Oncol 18 (1): 49–57
Gershenson DM (2010) The heterogeneity of epithelial ovarian cancer: getting it right. Cancer 116 (6): 1400–1402
Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, Deavers M, Malpica AL, Kavanagh JJ (2009) Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol 114 (1): 48–52
Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, Nikrui N, Tamimi HK, Cain JM, Greer BE, Fuller Jr AF (1996) Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol 60 (3): 412–417
Ho CM, Huang YJ, Chen TC, Huang SH, Liu FS, Chang Chien CC, Yu MH, Mao TL, Wang TY, Hsieh CY (2004) Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol 94 (1): 197–203
International Federation of Gynecology and Obstetrics (1987) Changing in definitions of clinical staging for carcinoma of the cervix and ovary. Am J Obstet Gynecol 156: 263–264
Kurman RJ, Shih IeM (2008) Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 27 (2): 151–160
Kurman RJ, Shih IeM (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34 (3): 433–443
Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IeM (2008) Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol 198 (4): 351–356
Pectasides D, Fountzilas G, Aravantinos G, Kalofonos HP, Efstathiou E, Salamalekis E, Farmakis D, Skarlos D, Briasoulis E, Economopoulos T, Dimopoulos MA (2005) Advanced stage mucinous epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol 97 (2): 436–441
Sehouli J, Könsgen D, Mustea A, Oskay-Ozcelik G, Katsares I, Weidemann H, Lichtenegger W (2003) [‘IMO’- intraoperative mapping of ovarian cancer]. Zentralbl Gynakol 125 (3–4): 129–135
Sehouli J, Richter R, Braicu EI, Bühling KJ, Bahra M, Neuhaus P, Lichtenegger W, Fotopoulou C (2010a) Role of secondary cytoreductive surgery in ovarian cancer relapse: who will benefit? A systematic analysis of 240 consecutive patients. J Surg Oncol 102 (6): 656–662
Sehouli J, Savvatis K, Braicu EI, Schmidt SC, Lichtenegger W, Fotopoulou C (2010b) Primary versus interval debulking surgery in advanced ovarian cancer: results from a systematic single-center analysis. Int J Gynecol Cancer 20 (8): 1331–1340
Sehouli J, Senyuva F, Fotopoulou C, Neumann U, Denkert C, Werner L, Gülten OO (2009) Intra-abdominal tumour dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol 99 (7): 424–427
Shih IeM, Kurman RJ (2004) Ovarian tumourigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol 164 (5): 1511–1518
Shih IeM, Kurman RJ (2005) Molecular pathogenesis of ovarian borderline tumours: new insights and old challenges. Clin Cancer Res 11 (20): 7273–7279
Singer G, Kurman RJ, Chang H-W, Cho SKR, Shih I-M (2002) Diverse tumourigenic pathways in ovarian serous carcinoma. Am J Pathol 160: 1223–1228
Vang R, Shih IeM, Kurman RJ (2009) Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol 16 (5): 267–282
Wimberger P, Wehling M, Lehmann N, Kimmig R, Schmalfeldt B, Burges A, Harter P, Pfisterer J, du Bois A (2010) Influence of residual tumour on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group). Ann Surg Oncol 17 (6): 1642–1648
Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA (2010) Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer 117 (3): 554–562
Acknowledgements
This study has been carried out with the approval of the local ethics committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Synopsis
Type I ovarian, fallopian tube and peritoneal cancer patients defined as low-grade serous, endometrioid, clear cell and mucinous tumours as well as transitional carcinomas are (i) significantly younger, (ii) present at significantly earlier stages, (iii) have a lower incidence of ascites and lymph node involvement and (iv) have significantly better clinical and surgical outcomes compared with patients with type II tumours, that is, with high-grade serous, endometrioid or undifferentiated tumours, or carcinosarcomas. Nevertheless, when considering only the subgroup of patients with advanced FIGO stages III and IV, the histological type does not show any significant prognostic value when considering either survival or surgical outcome.
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Braicu, EI., Sehouli, J., Richter, R. et al. Role of histological type on surgical outcome and survival following radical primary tumour debulking of epithelial ovarian, fallopian tube and peritoneal cancers. Br J Cancer 105, 1818–1824 (2011). https://doi.org/10.1038/bjc.2011.455
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.455
Keywords
This article is cited by
-
Fallopian tube lesions as potential precursors of early ovarian cancer: a comprehensive proteomic analysis
Cell Death & Disease (2023)
-
Outcome quality standards in advanced ovarian cancer surgery
World Journal of Surgical Oncology (2020)
-
ARID1A mutation sensitizes most ovarian clear cell carcinomas to BET inhibitors
Oncogene (2018)
-
Cancer of the ovary, fallopian tube, and peritoneum: a population-based comparison of the prognostic factors and outcomes
Journal of Cancer Research and Clinical Oncology (2017)
-
The effect of time on racial differences in epithelial ovarian cancer (OVCA) diagnosis stage, overall and by histologic subtypes: a study of the National Cancer Database
Cancer Causes & Control (2016)