Abstract
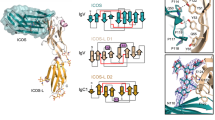
Optimal immune responses require both an antigen-specific and a co-stimulatory signal. The shared ligands B7-1 and B7-2 on antigen-presenting cells deliver the co-stimulatory signal through CD28 and CTLA-4 on T cells. Signalling through CD28 augments the T-cell response, whereas CTLA-4 signalling attenuates it. Numerous animal studies1,2 and recent clinical trials3,4 indicate that manipulating these interactions holds considerable promise for immunotherapy. With the consequences of these signals well established, and details of the downstream signalling events emerging5,6,7, understanding the molecular nature of these extracellular interactions becomes crucial. Here we report the crystal structure of the human CTLA-4/B7-1 co-stimulatory complex at 3.0 Å resolution. In contrast to other interacting cell-surface molecules, the relatively small CTLA-4/B7-1 binding interface exhibits an unusually high degree of shape complementarity. CTLA-4 forms homodimers through a newly defined interface of highly conserved residues. In the crystal lattice, CTLA-4 and B7-1 pack in a strikingly periodic arrangement in which bivalent CTLA-4 homodimers bridge bivalent B7-1 homodimers. This zipper-like oligomerization provides the structural basis for forming unusually stable signalling complexes at the T-cell surface, underscoring the importance of potent inhibitory signalling in human immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lenschow, D. J., Walunas, T. L. & Bluestone, J. A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Greenfield, E. A., Nguyen, K. A. & Kuchroo, V. K. CD28/B7 costimulation: a review. Crit. Rev. Immunol. 18, 389–418 (1998).
Guinan, E. C. et al. Transplantation of anergic histoincompatible bone marrow allografts. N. Engl. J. Med. 340, 1704–1714 (1999).
Abrams, J. R. et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J. Exp. Med. 192, 681–694 (2000).
van der Merwe, P. A., Davis, S. J., Shaw, A. S. & Dustin, M. L. Cytoskeletal polarization and redistribution of cell-surface molecules during T cell antigen recognition. Semin. Immunol. 12, 5–21 (2000).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Lee, K.-M. et al. Molecular basis of T cell inactivation by CTLA-4. Science 282, 2263–2266 (1998).
Ostrov, D. A., Shi, W., Schwartz, J.-C. D., Almo, S. C. & Nathenson, S. G. Structure of murine CTLA4 and its role in modulating T cell responsiveness. Science 290, 816–819 (2000).
Metzler, W. J. et al. Solution structure of human CTLA4 and delineation of a CD80/CD86 binding site conserved in CD28. Nature Struct. Biol. 4, 527–531 (1997).
Ikemizu, S. et al. Structure and dimerization of a soluble form of B7-1. Immunity 12, 51–60 (2000).
Collaborative computational project No. 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–776 (1994).
van der Merwe, P. A., Bodian, D. L., Daenke, S., Linsley, P. & Davis, S. J. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 185, 393–403 (1997).
Peach, R. J. et al. Both extracellular immunoglobulin-like domains of CD80 contain residues critical for binding T cell surface receptors CTLA-4 and CD28. J. Biol. Chem. 8, 21181–21187 (1995).
Janin, J. & Chothia, C. The structure of protein-protein recognition sites. J. Biol. Chem. 265, 16027–16030 (1990).
Jones, E. Y., Davis, S. J., Williams, A. F., Harlos, K. & Stuart, D. I. Crystal structure at 2. 8 Å resolution of a soluble form of the cell adhesion molecule CD2. Nature 360, 232–239 (1992).
Bodian, D. L., Jones, E. Y., Harlos, K., Stuart, D. I. & Davis, S. J. Crystal structure of the extracellular region of the human cell adhesion molecule CD2 at 2.5 Å resolution. Structure 15, 755–766 (1994).
Wang, J.-h. et al. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell 7, 791–803 (1999).
van Raaij, M. J., Chouin, E., van der Zandt, H., Bergelson, J. M. & Cusack, S. Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1. 7 Å resolution. Struct. Fold Des. 8, 1147–1155 (2000).
Lawrence, M. C. & Colman, P. M. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234, 946–950 (1993).
Garboczi, D. N. et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature 384, 134–141 (1996).
Boyington, J. C., Motyka, S. A., Schuck, P., Brooks A. G. & Sun, P. D. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature 405, 537–543 (2000).
Shapiro, L. et al. Structural basis of cell–cell adhesion by cadherins. Nature 374, 327–337 (1995).
Davis, S. J., Ikemizu, S., Wild, M. K. & van der Merwe, P. A. CD2 and the nature of protein interactions mediating cell-cell recognition. Immunol. Rev. 163, 217–236 (1998).
Griffin, M. D. et al. Blockade of T cell activation using a surface-linked single-chain antibody to CTLA-4 (CD152). J. Immunol. 164, 4433–4442 (2000).
Bachmann, M. F., Kohler, G., Ecabert, B., Mak, T. W. & Kopf, M. Lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol. 163, 1128–1131 (1999).
Somers, W. S., Tang, J., Shaw, G. D. & Camphausen, R. T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to sLex and PSGL-1. Cell 103, 467–479 (2000).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Brunger, A. T. et al. Crystallography and NMR system (CNS): a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Carson, M. Ribbons 2.0. J. Appl. Crystallogr. 24, 958–961 (1991).
Esnouf, R. M. An extensively modified version of Molscript that includes enhanced colouring capabilities. J. Mol. Graph. 15, 132–134 (1997).
Acknowledgements
We thank R. Zollner and his colleagues for large scale cell culture, and D. I. Stuart and E. Y. Jones for helpful comments. We also thank the staff at Advanced Light Source for assistance with data collection. S.I. and S.J.D. are supported by the Wellcome Trust and the Human Frontier Science Programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stamper, C., Zhang, Y., Tobin, J. et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 410, 608–611 (2001). https://doi.org/10.1038/35069118
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35069118
This article is cited by
-
Immune evasion in cell-based immunotherapy: unraveling challenges and novel strategies
Journal of Biomedical Science (2024)
-
Costimulatory receptors in the channel catfish: CD28 family members and their ligands
Immunogenetics (2024)
-
The homodimer interfaces of costimulatory receptors B7 and CD28 control their engagement and pro-inflammatory signaling
Journal of Biomedical Science (2023)
-
Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy
Journal of Hematology & Oncology (2023)
-
The engineered CD80 variant fusion therapeutic davoceticept combines checkpoint antagonism with conditional CD28 costimulation for anti-tumor immunity
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.