Abstract
Purpose. The presence of single nucleotide polymorphisms (SNPs) has been reported for multidrug resistance-associated protein 2 (MRP2/ABCC2). The purpose of the current study was to characterize the localization, expression level, and function of MRP2 variants.
Methods. The expression and cellular localization of the wild-type and three kinds of reported SNP variants of MRP2 molecules were analyzed in LLC-PK1 cells after infection with the recombinant Tet-off adenoviruses. Their function was determined by using the isolated membrane vesicles from the infected LLC-PK1 cells.
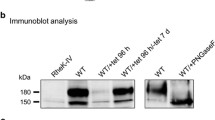
Results. The transport activity for E217βG, LTC4, and DNP-SG, normalized by the expression level of MRP2, was similar between the wild-type, V417I, and A1450T MRP2s. The transport activity of S789F MRP2 was slightly higher than that of wild-type MRP2. However, the expression level of S789F and A1450T MRP2 proteins was significantly lower compared with the wild-type and V417I MRP2. In addition, although the wild-type and V417I MRP2 were exclusively localized in the apical membrane, S789F and A1450T MRP2 were located in the apical membrane and also in the intracellular compartment.
Conclusions. These results suggest that the most frequently observed V417I substitution may not affect the in vivo function of MRP2, whereas the much less frequently observed S789F and A1450T may be associated with the reduced in vivo function.
Similar content being viewed by others
references
P. Anzenbacher and E. Anzenbacherova. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. 58:737-747 (2001).
J. A. Goldstein. Clinical relevance of genetic polymorphisms in the human CYP2C subfamily. Br. J. Clin. Pharmacol. 52:349-355 (2001).
J. H. Lin and A. Y. Lu. Interindividual variability in inhibition and induction of cytochrome P450 enzymes. Annu. Rev. Pharmacol. Toxicol. 41:535-567 (2001).
S. Hoffmeyer, O. Burk, O. von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97:3473-3478 (2000).
R. G. Tirona, B. F. Leake, G. Merino, and R. B. Kim. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European-and African-Americans. J. Biol. Chem. 276:35669-35675 (2001).
T. Nozawa, M. Nakajima, I. Tamai, K. Noda, J. Nezu, Y. Sai, A. Tsuji, and T. Yokoi. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J. Pharmacol. Exp. Ther. 302:804-813 (2002).
M. F. Fromm. The influence of MDR1 polymorphisms on P-glycoprotein expression and function in humans. Adv. Drug Deliv. Rev. 54:1295-1310 (2002).
H. Suzuki and Y. Sugiyama. Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Adv. Drug Deliv. Rev. 54:1311-1331 (2002).
H. Suzuki and Y. Sugiyama. Transporters for bile acids and organic anions. Pharm. Biotechnol. 12:387-439 (1999).
D. Keppler and J. Konig. Hepatic secretion of conjugated drugs and endogenous substances. Semin. Liver Dis. 20:265-272 (2000).
H. Suzuki and Y. Sugiyama. Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur. J. Pharm. Sci. 12:3-12 (2000).
Y. Gotoh, H. Suzuki, S. Kinoshita, T. Hirohashi, Y. Kato, and Y. Sugiyama. Involvement of an organic anion transporter (canalicular multispecific organic anion transporter/multidrug resistance-associated protein 2) in gastrointestinal secretion of glutathione conjugates in rats. J. Pharmacol. Exp. Ther. 292:433-439 (2000).
C. G. Dietrich, D. R. de Waart, R. Ottenhoff, I. G. Schoots, and R. P. Elferink. Increased bioavailability of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol. Pharmacol. 59:974-980 (2001).
L. Payen, L. Sparfel, A. Courtois, L. Vernhet, A. Guillouzo, and O. Fardel. The drug efflux pump MRP2: regulation of expression in physiopathological situations and by endogenous and exogenous compounds. Cell Biol. Toxicol. 18:221-233 (2002).
R. O. Elferink and A. K. Groen. Genetic defects in hepatobiliary transport. Biochim. Biophys. Acta 1586:129-145 (2002).
P. Borst and R. O. Elferink. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71:537-592 (2002).
V. Keitel, J. Kartenbeck, A. T. Nies, H. Spring, M. Brom, and D. Keppler. Impaired protein maturation of the conjugate export pump multidrug resistance protein 2 as a consequence of a deletion mutation in Dubin-Johnson syndrome. Hepatology 32:1317-1328 (2000).
K. Hashimoto, T. Uchiumi, T. Konno, T. Ebihara, T. Nakamura, M. Wada, S. Sakisaka, F. Maniwa, T. Amachi, K. Ueda, and M. Kuwano. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology 36:1236-1245 (2002).
S. Ito, I. Ieiri, M. Tanabe, A. Suzuki, S. Higuchi, and K. Otsubo. Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cMOAT, in healthy Japanese subjects. Pharmacogenetics 11:175-184 (2001).
M. Itoda, Y. Saito, A. Soyama, M. Saeki, N. Murayama, S. Ishida, K. Sai, M. Nagano, H. Suzuki, Y. Sugiyama, S. Ozawa, and J. Sawada. Polymorphisms in the ABCC2 (cMOAT/MRP2) gene found in 72 established cell lines derived from Japanese individuals: an association between single nucleotide polymorphisms in the 5′-untranslated region and exon 28. Drug Metab. Dispos. 30:363-364 (2002).
M. Wada, S. Toh, K. Taniguchi, T. Nakamura, T. Uchiumi, K. Kohno, I. Yoshida, A. Kimura, S. Sakisaka, Y. Adachi, and M. Kuwano. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum. Mol. Genet. 7:203-207 (1998).
K. Kobayashi, Y. Sogame, H. Hara, and K. Hayashi. Mechanism of glutathione S-conjugate transport in canalicular and basolateral rat liver plasma membranes. J. Biol. Chem. 265:7737-7741 (1990).
H. Mizuguchi and M. A. Kay. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum. Gene Ther. 9:2577-2583 (1998).
H. Mizuguchi and M. A. Kay. A simple method for constructing E1-and E1/E4-deleted recombinant adenoviral vectors. Hum. Gene Ther. 10:2013-2017 (1999).
M. Muller, C. Meijer, G. J. Zaman, P. Borst, R. J. Scheper, N. H. Mulder, E. G. de Vries, and P. L. Jansen. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc. Natl. Acad. Sci. USA 91:13033-13037 (1994).
T. Hirohashi, H. Suzuki, X. Y. Chu, I. Tamai, A. Tsuji, and Y. Sugiyama. Function and expression of multidrug resistance-associated protein family in human colon adenocarcinoma cells (Caco-2). J. Pharmacol. Exp. Ther. 292:265-270 (2000).
R. Evers, M. Kool, L. van Deemter, H. Janssen, J. Calafat, L. C. Oomen, C. C. Paulusma, R. P. Oude Elferink, F. Baas, A. H. Schinkel, and P. Borst. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J. Clin. Invest. 101:1310-1319 (1998).
Y. Cui, J. Konig, J. K. Buchholz, H. Spring, I. Leier, and D. Keppler. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol. Pharmacol. 55:929-937 (1999).
K. Ito, H. Suzuki, and Y. Sugiyama. Charged amino acids in the transmembrane domains are involved in the determination of the substrate specificity of rat Mrp2. Mol. Pharmacol. 59:1077-1085 (2001).
S. Kajihara, A. Hisatomi, T. Mizuta, T. Hara, I. Ozaki, I. Wada, and K. Yamamoto. A splice mutation in the human canalicular multispecific organic anion transporter gene causes Dubin-Johnson syndrome. Biochem. Biophys. Res. Commun. 253:454-457 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirouchi, M., Suzuki, H., Itoda, M. et al. Characterization of the Cellular Localization, Expression Level, and Function of SNP Variants of MRP2/ABCC2. Pharm Res 21, 742–748 (2004). https://doi.org/10.1023/B:PHAM.0000026422.06207.33
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000026422.06207.33