Abstract
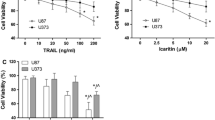
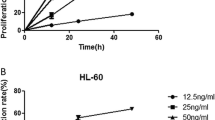
There are many factors contributing to the resistance to TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand)-induced apoptosis. However, it is not clear whether the mechanism of resistance to TRAIL is constitutive or inductive. Therefore, the purpose of this study was to investigate the resistant mechanisms to TRAIL at different levels in the apoptotic pathway. The human T-lymphoblastic leukemic CEM cell line showed more resistant to TRAIL-induced apoptosis compared with the human chronic myeloid leukemic K562 cell line. Lower level of constitutive caspase-8 expression in the CEM cell line led to a poor response to both TRAIL-induced activation of caspase-3 and reduction in the mitochondrial membrane potential (ΔΨm). There was no significant difference in the constitutive levels of NF-κB in CEM and K562 cell lines. However, CEM cells showed a faster response to TRAIL-induced NF-κB activation than K562 cells. TRAIL-induced regulation of Bcl-2 family of proteins included an up-regulation in Bcl-2/Bcl-XL and a down-regulation in Bax. IAPs, such as XIAP, cIAP-1, cIAP-2 and Survivin were all up-regulated during the treatment with TRAIL. In summary, our data suggest that the leukemic cells resistance to TRAIL-induced apoptosis might be due to the deficiency in the constitutive caspase-8 expression. Development of potential resistance to apoptosis by TRAIL can occur in both TRAIL-resistant and TRAIL-sensitive leukemic cells.
Similar content being viewed by others
References
Ashkenazi A, Dixit VM. Death receptors: Signaling and modulation. Science 1998; 281: 1305–1308.
Griffith TS, Lynch DH. TRAIL: A molecule with multiple receptors and control mechanisms.Curr Opin Immunol 1998; 10: 559–563.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.
Wei MC, Lindsten T, Mootha VK, et al. BID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 2000; 24: 2060–2071.
Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Yamada H, Tada-Oikawa S, Uchida A, Kawanishi S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun 1999; 265: 130–133.
Hinz S, Trauzold A, Boenicke L, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95-and TRAIL-receptor-mediated apoptosis.Oncogene 2000; 19: 5477–5486.
Jia L, Patwari Y, Kelsey SM, Newland AC. Trail-induced apoptosis in Type I leukemic cells is not enhanced by overexpression of Bax. Biochem Biophys Res Commun 2001; 283: 1037–1045.
Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Ozoren N, El-Deiry WS. Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia 2002; 4: 551–557
Grotzer MA, Eggert A, Zuzak TJ, et al. Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene 2000; 19: 4604–4610.
Oya M, Ohtsubo M, Takayanagi A, Tachibana M, Shimizu N, Murai M. Constitutive activation of nuclear factor-?B prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene 2001; 20: 3888–3896.
Mitsiades CS, Treon SP, Mitsiades N, et al. TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug resistance in multiple myeloma: Therapeutic applications. Blood 2001; 98: 795–804.
Thomas RP, Farrow BJ, Kim S, May MJ, Hellmich MR, Evers BM. Selective targeting of the nuclear factor-?B pathway enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated pancreatic cancer cell death. Surgery 2002; 132: 127–134.
Dalen H, Neuzil J. alpha-Tocopheryl succinate sensitises a T lymphoma cell line to TRAIL-induced apoptosis by suppressing NF-kappaB activation. Br J Cancer 2003; 88: 153–158.
Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 2002; 21: 2283–2294
Jia L, Patwari Y, Kelsey SM, et al. Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene 2003; 22: 1589–1599.
Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene 2003; 22: 3842–3852.
Mitsiades N, Mitsiades CS, Poulaki V, et al. Biologic sequelae of nuclear factor-?B blockade in multiple myeloma: Therapeutic applications. Blood 2002; 99: 4079–4086.
Jia L, Srinivasula SM, Liu FT, et al. Apaf-1 protein deficiency confers resistance to cytochrome c-dependent apoptosis in human leukemic cells. Blood 2001; 98: 414–421.
Suliman A, Lam A, Datta R, Srivastava RK. Intracellular mechanisms of TRAIL: Apoptosis through mitochondrial-dependent and-independent pathways. Oncogene 2001; 20: 2122–2133.
Jia L, Kelsey SM, Grahn MF, Jiang XR, Newland AC. Increased activity and sensitivity of mitochondrial respiratory enzymes to tumor necrosis factor alpha-mediated inhibition is associated with increased cytotoxicity in drug-resistant leukemic cell lines. Blood 1996; 87: 2401–2410.
Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 1998; 17: 1675–1687
Fulda S, Kufer MU, Meyer E, van Valen F, Dockhorn-Dworniczak B, Debatin KM. Sensitization for death receptoror drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 2001; 20: 5865–5877.
Lee KY, Park JS, Jee YK, Rosen GD. Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappakB activation. Exp Mol Med 2002; 34: 462–468.
Leverkus M, Sprick MR, Wachter T, et al. Proteasome inhibition results in TRAIL sensitization of primary keratinocytes by removing the resistance-mediating block of effector caspase maturation. Mol Cell Biol 2003; 23: 777–790.
Sayers TJ, Brooks AD, Koh CY, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood 2003; 102: 303–310.