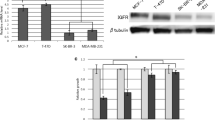
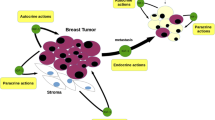
Abstract
Once it was recognized that breast tumor growth was stimulated by estrogens, successfultherapeutic strategies based on depriving the tumor of this hormone were developed. Sincethe growth stimulatory properties of the estrogens are governed by the estrogen receptor (ER),4understanding the mechanisms that activate ER are highly relevant. In addition to estrogens,peptide growth factors can also activate the ER. The insulin-like growth factors (IGFs) arepotent mitogens for ER-positive breast cancer cell lines. This review will examine the evidencefor interaction between these two pathways. The IGFs can activate the ER, while ERtranscriptionally regulates genes required for IGF action. Moreover, blockade of ER function can inhibitIGF-mediated mitogenesis and interruption of IGF action can similarly inhibit estrogenicstimulation of breast cancer cells. Taken together, these observations suggest that the twogrowth regulatory pathways are tightly linked and that a further understanding of the mechanismof this crosstalk could lead to new therapeutic strategies in breast cancer.
Similar content being viewed by others
REFERENCES
V. C. Jordan (1996). Tamoxifen, A Guide for Clinicans and Patients, PRR, Huntington, New York.
V. C. Jordan (1999). Estrogen receptor as a target for the prevention of breast cancer. J. Lab. Clin. Med. 133:408–414.
D. J. Mangelsdorf, C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon (1995). The nuclear receptor superfamily: The second decade. Cell 83:835–839.
G. G. Kuiper, E. Enmark, M. Pelto-Huikko, S. Nilsson, and J. A. Gustafsson (1996). Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U.S.A. 93:5925–5930.
L. A. Paige, D. J. Christensen, H. Gron, J. D. Norris, E. B. Gottlin, K. M. Padilla, C. Y. Chang, L. M. Ballas, P. T. Hamil ton, D. P. McDonnell, and D. M. Fowlkes (1999). Estrogen receptor (ER) modulators each induce distinct conformational changes in ER alpha and ER beta. Proc. Natl. Acad. Sci. U.S.A. 96:3999–4004.
H. Shibata, T. E. Spencer, S. A. Onate, G. Jenster, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley (1997). Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog. Horm. Res. 52:141–164.
M. G. Parker (1998). Transcriptional activation by oestrogen receptors. Biochem. Soc. Symp. 63:45–50.
M. D. PlanasSilva and R. A. Weinberg (1997). Estrogen-dependent cyclin E-cdk2 activation through p21 redistribution. Mol. Cell. Biol. 17:4059–4069.
R. F. Power, S. K. Mani, J. Codina, O. M. Conneely, and B.W. O'Malley (1991). Dopaminergic and ligand-independent activation of steroid hormone receptors. Science 254: 1636–1639.
D. M. Ignar-Trowbridge, K. G. Nelson, M. C. Bidwell, S. W. Curtis, T. F. Washburn, J. A. McLachlan, K. S. Korach (1992). Coupling of dual signaling pathways: Epidermal growth factor action involves the estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 89:4658–4662.
D. M. Ignar-Trowbridge, M. Pimentel, M. G. Parker, J. A. McLachlan, and K. S. Korach (1996). Peptide growth factor cross-talk with the estrogen receptor requires the A/B domain and occurs independently of protein kinase C or estradiol. Endocrinology 137:1735–1744.
D. M. Ignar-Trowbridge, C. T. Teng, K. A. Ross, M. G. Parker, K. S. Korach, and J. A. McLachlan (1993). Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Mol. Endocrinol. 7:992–998.
H. S. Cho, S. M. Aronica, and B. S. Katzenellenbogen (1994). Regulation of progesterone receptor gene expression in MCF-7 breast cancer cells—a comparison of the effects of cyclic adenosine 3',5'-monophosphate, estradiol, insulin-like growth factor-I, and serum factors. Endocrinology 134:658–664.
B. S. Katzenellenbogen and M. J. Norman (1990). Multihor monal regulation of the progesterone receptor in MCF-7 human breast cancer cells: Interrelationships among insulin/insulin-like growth factor-I, serum, and estrogen. Endocrinology 126:891–898.
B. S. Katzenellenbogen (1996). Estrogen receptors: Bioactivities and interactions with cell signaling pathways. Biol. Reprod. 54:287–293.
C. Patrone, Z. Q. Ma, G. Pollio, P. Agrati, M. G. Parker, and A. Maggi (1996). Cross-coupling between insulin and estrogen receptor in human neuroblastoma cells. Mol. Endocrinol. 10:499–507.
M. L. Panno, M. Salerno, V. Pezzi, D. Sisci, M. Maggiolini, L. Mauro, E. G. Morrone, and S. Ando (1996). Effect of oestra diol and insulin on the proliferative pattern and on oestrogen and progesterone receptor contents in MCF-7 cells. J. Cancer Res. Clin. Oncol. 122:745–749.
A. V. Lee, C. N. Weng, J. G. Jackson, and D. Yee (1997). Activation of estrogen receptor-mediated gene transcription by IGF-I in human breast cancer cells. J. Endocrinol. 152:39–47.
A. V. Lee, J. G. Jackson, J. G. Gooch, S. G. Hilsenbeck, E. Coronado-Heinsohn, C. K. Osborne, and D. Yee (1999). Enhancement of the insulin-like growth factor pathway by estrogen in human breast cancer cells. Mol. Endocrinol. 13:787–796.
G. Freiss, C. Puech, and F. Vignon (1998). Extinction of insulin-like growth factor-I mitogenic signaling by antiestrogen-stimulated Fas-associated protein tyrosine phosphatase-1 in human breast cancer cells. Mol. Endocrinol. 12:568–579.
M. Salerno, D. Sisci, L. Mauro, M. A. Guvakova, S. Ando, and E. Surmacz (1999). Insulin receptor substrate 1 is a target for the pure antiestrogen ICI 182,780 in breast cancer cells. Int. J. Cancer 81:299–304.
M. A. Guvakova and E. Surmacz (1997). Tamoxifen interferes with the insulin-like growth factor I receptor (IGF-IR) signaling pathway in breast cancer cells. Cancer Res. 57:2606–2610.
G. Freiss, H. Rochefort, and F. Vignon (1990). Mechanisms of 4-hydroxytamoxifen anti-growth factor activity in breast cancer cells: Alterations of growth factor receptor binding sites and tyrosine kinase activity. Biochem. Biophys. Res. Com mun. 173:919–926.
M. K. El-Tanani and C. D. Green (1997). Two separate mecha-nisms for ligand-independent activation of the estrogen receptor. Mol. Endocrinol. 11:928–937.
S. F. Arnold, J. D. Obourn, H. Jaffe, and A. C. Notides (1994). Serine 167 is the major estradiol-induced phosphorylation site on the human estrogen receptor. Mol. Endocrinol. 8:1208–1214.
S. Ali, D. Metzger, J. M. Bornert, and P. Chambon (1993). Modulation of transcriptional activation by ligand-dependent phosphorylation of the human oestrogen receptor A/B region. EMBO J. 12:1153–1160.
P. Le Goff, M. M. Montano, D. J. Schodin, and B. S. Katzenellenbogen (1994). Phosphorylation of the human estrogen recep tor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J. Biol. Chem. 269:4458–4466.
S. Kato, H. Endoh, Y. Masuhiro, T. Kitamoto, S. Uchiyama, H. Sasaki, S. Masushige, Y. Gotoh, E. Nishida, H. Kawashima, D. Metzger, and P. Chambon (1995). Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494.
G. Bunone, P. A. Briand, R. J. Miksicek, and D. Picard (1996). Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 15:2174–2183.
S. M. Aronica and B. S. Katzenellenbogen (1993). Stimulation of estrogen receptor-mediated transcription and alteration in the phosphorylation state of the rat uterine estrogen receptor by estrogen, cyclic adenosine monophosphate, and insulin-like growth factor-I. Mol. Endocrinol. 7:743–752.
C. Patrone, E. Gianazza, S. Santagati, P. Agrati, and A. Maggi (1998). Divergent pathways regulate ligand-independent activation of ER alpha in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol. Endocrinol. 12:835–841.
G. B. Tremblay, A. Tremblay, N. G. Copeland, D. J. Gilbert, N. A. Jenkins, F. Labrie, and V. Giguere (1997). Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol. Endocrinol. 11:353–365.
P. Webb, P. Nguyen, J. Shinsako, C. Anderson, W. Feng, M. P. Nguyen, D. Chen, S. M. Huang, S. Subramanian, E. McKinerney, B. S. Katzenellenbogen, M. R. Stallcup, and P. J. Kushner (1998). Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol. Endocrinol. 12:1605–1618.
Z. Q. Ma, S. Santagati, G. Patrone, G. Pollio, E. Vegeto, and A. Maggi (1994). Insulin-like growth factors activate estrogen receptor to control the growth and differentiation of the human insulinneuroblastoma cell line SK-ER3. Mol. Endocrinol. 8:910–918.
C. Patrone, Z. Q. Ma, S. Santagati, E. Vegeto, and A. Maggi (1995). Ras mediates the insulin-depenedent activation of the unliganded estrogen receptor in human neuroblastoma cells. Progr. 77th Ann. Meeting of the Endocrine Society, Washington, D.C. (Abstract) p. 1–469.
R. M. Zwijsen, E. Wientjens, R. Klompmaker, J. van der Sman, R. Bernards, and R. J. Michalides (1997). CDK-independent activation of estrogen receptor by cyclin D1. Cell 88:405–415.
E. Neuman, M. H. Ladha, N. Lin, T. M. Upton, S. J. Miller, J. DiRenzo, R. G. Pestell, P.W. Hinds, S. F. Dowdy, M. Brown, and M. E. Ewen (1997). Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell. Biol. 17:5338–5347.
R. M. Zwijsen, R. S. Buckle, E. M. Hijmans, C. J. Loomans, and R. Bernards (1998). Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 12:3488–3498.
C. McMahon, T. Suthiphongchai, J. DiRenzo, and M. E. Ewen (1999). P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc. Natl. Acad. Sci. U.S.A. 96:5382–5387.
B. Dufourny, J. Albas, H. A. van Teefelen, F. M. van Schaik, B. van der Burg, and P. H. Steenbergh, J. S. Sussenbach (1997). Mitogenic signaling of insulin-like growth factor I in MCF-7 human breast cancer cells requires phosphatidylinositol 3-kinase and is independent of mitogen-activated protein kinase. J. Biol. Chem. 272:31163–31171.
W. Ruan, R. Catanese, R. Wieczorek, M. Feldman, and D. L. Kleinberg (1995). Estradiol enhances the stimulatory effect of insulin-like growth factor-I (IGF-I) on mammary development and growth-hormone-induced IGF-I messenger ribonucleic acid. Endocrinology 136:1296–1302.
R. G. Richards, R. P. DiAugustine, P. Petrusz, G. C. Clark, and J. Sebastian (1996). Estradiol stimulates tyrosine phosphor ylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc. Natl. Acad. Sci. U.S.A. 93:12002–12007.
D. Kleinman, M. Karas, C. T. Roberts, Jr., D. LeRoith, M. Phillip, Y. Segev, J. Levy, and Y. Sharoni (1995). Modulation of insulin-like growth factor I (IGF-I) receptors and membrane-associated IGF-binding proteins in endometrial cancer cells by estradiol. Endocrinology 136:2531–2537.
A. Stewart, B. Westley, and F. May (1992). Modulation of the proliferative response of breast cancer cells to growth factors by oestrogen. Brit. J. Cancer 66:640–648.
B. Katzenellenbogen, and M. J. Norman (1990). Multihormonal regulation of the progesterone receptor in MCF-7 human breast cancer cells: Interrelationships among insulin/insulin-like growth factor-I, serum, and estrogen. Endocrinology 126: 891–898.
A. E. Wakeling, E. Newboult, and S. W. Peters (1989). Effects of antioestrogens on the proliferation of MCF-7 human breast cancer cells. J. Mol. Endocrinol. 2:225–234.
A. V. Lee, P. Darbre, and R. J. King (1994). Processing of insulin-like growth factor-II (IGF-II) by human breast cancer cells. Mol. Cell. Endocrinol. 99:211–220.
C. K. Osborne, E. B. Coronado, L. J. Kitten, C. I. Arteaga, S. A. W. Fuqua, K. Ramasharma, M. Marshall, and C. H. Li (1989). Insulin-like growth factor-II (IGF-II): A potential autocrine/paracrine growth factor for human breast cancer acting via the IGF-I receptor. Mol. Endocrinol. 3:1701–1709.
D. Yee, K. Cullen, S. Paik, J. Perdue, B. Hampton, A. Schwartz, M. Lippman, and N. Rosen (1988). Insulin-like growth factor II mRNA expression in human breast cancer. Cancer Res. 48:6691–6696.
M. Mathieu, F. Vignon, F. Capony, and H. Rochefort (1991). Estradiol down-regulates the mannose-6-phosphate/insulinneuroblastoma like growth factor-II receptor gene and induces cathepsin-D in breast cancer cells:Areceptor saturation mechanism to increase the secretion of lysosomal proenzymes. Mol. Endocrinol. 5:815–822.
W. McGuire, Jr., J. G. Jackson, J. A. Figueroa, S. A. Shimasaki, D. R. Powell, and D. Yee (1992). Regulation of insulin-like growth factor-binding protein (IGFBP) expression by breast cancer cells: Use of IGFBP-1 as an inhibitor of insulin-like growth factor action. J. Natl. Cancer Inst. 84:1336–1341.
A. V. Lee, J. G. Jackson, J. L. Gooch, S. G. Hilsenbeck, E. Coronado-Heinsohn, C. K. Osborne, and D. Yee (1999). Enhancement of insulin-like growth factor signaling in human breast cancer: Estrogen regulation of insulin receptor substrate-1 expression in vitro and in vivo. Mol. Endocrinol. 13:787–796.
R. G. Richards, M. P. Walker, J. Sebastian, and R. P. DiAugus tine (1998). Insulin-like growth factor-1 (IGF-1) receptor-insulin receptor substrate complexes in the uterus. Altered signaling response to estradiol in the IGF-1(m/m) mouse. J. Biol. Chem. 273:11962–11969.
X. F. Yang, W. G. Beamer, H. Huynh, and M. Pollak (1996). Reduced growth of human breast cancer xenografts in hosts homozygous for the lit mutation. Cancer Res. 56:1509–1511.
S. E. Hankinson, W. C. Willett, G. A. Colditz, D. J. Hunter, D. S. Michaud, B. Deroo, B. Rosner, F. E. Speizer, and M. Pollak (1998). Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 351:1393–1396.
M. N. Pollak (1998). Endocrine effects of IGF-I on normal and transformed breast epithelial cells: Potential relevance to strategies for breast cancer treatment and prevention. Breast Cancer Res. Treat. 47:209–217.
R. L. Rocha, S. G. Hilsenbeck, J. G. Jackson, C. L. van den Berg, C-N. Weng, A. V. Lee, and D. Yee (1997). Insulin-like growth factor binding protein-3 and insulin receptor substrateand 1 in breast cancer: correlation with clinical parameters and disease-free survival. Clin. Cancer Res. 3:103–109.
H. Huynh, X. Yang, and M. Pollak (1996). Estradiol and anties-trogens regulate a growth inhibitory insulin-like growth factor-binding protein 3 autocrine loop in human breast cancer cells. J. Biol. Chem. 271:1016–1021.
J. A. Figueroa, J. G. Jackson, W. L. McGuire, R. F. Krywicki, and D. Yee (1993). Expression of insulin-like growth factor binding proteins in human breast cancer correlates with estro gen receptor status. J. Cell Biochem. 52:196–205.
G. Freiss, H. Rochefort, and F. Vignon (1990). Mechanisms of 4-hydroxytamoxifen anti-growth factor activity in breast cancer cells: Alterations of growth factor receptor binding sites and tyrosine kinase activity. Biochem. Biophys. Res. Com mun. 3:919–926.
G. Freiss and F. Vignon (1994). Antiestrogens increase protein tyrosine phosphatase activity in human breast cancer cells. Mol. Endocrinol. 8:1389–1396.
R. Daly, W. Harris, D. Wang, and P. Darbre (1991). Autocrine production of insulin like growth factor-II using an inducible expression system results in reduced estrogen sensitivity of MCF-7 human breast cancer cells. Cell Growth Differ. 2:457–464.
K. Cullen, M. Lippman, D. Chow, S. Hill, N. Rosen, and J. Zwiebel (1992). Insulin-like growth factor-II over expression in MCF-7 cells induces phenotypic chances associated with malignant progression. Mol. Endocrinol. 6:91–100.
A. V. Lee, M. Toi, and D. Yee (1994). Tamoxifen resistant MCF-7 cells exhibit increased expression of insulin-like growth factor-II. Breast Cancer Res. Treat. 32S:57.
L. R. Wiseman, M. D. Johnson, A. E. Wakeling, A. E. Lykkesfeldt, F. E. May, and B. R. Westley (1993). Type I IGF receptor and acquired tamoxifen resistance in oestrogen-responsive human breast cancer cells. Eur. J. Cancer 29A:2256–2264.
M. A. Guvakova and E. Surmacz (1997). Overexpressed IGFI receptors reduce estrogen growth requirements, enhance sur vival, and promote E-cadherin-mediated cell-cell adhesion in human breast cancer cells. Exper. Cell Res. 231:149–162.
M. R. Daws, B. R. Westley, and F. E. May (1996). Paradoxical effects of overexpression of the type I insulin-like growth factor (IGF) receptor on the responsiveness of human breast cancer cells to IGFs and estradiol. Endocrinology 137:1177–1186.
E. Surmacz and J-L. Burgard (1995). Overexpression of IRS-1 in the human breast cancer cell line MCF-7 induces loss of estrogen requirements for growth and transformation. Clin. Cancer Res. 1:1429–1436.
D. McCotter, H.W. van den Berg, M. Boylan, and B. McKibben (1996). Changes in insulin-like growth factor-I receptor expression and binding protein secretion associated with tamoxifen resistance and estrogen independence in human breast cancer cells in vitro. Cancer Lett. 99:239–245.
M. K. Dougherty, L. M. Schumaker, V. C. Jordan, and M. J. C. Ellis (1999). Status of the insulin-like growth factor network in an estrogen receptor negative T47D breast cancer cell clone. Proc. AACR 40:4042.
A. V. Lee, R. L. Guler, S. Oesterrecih, L. A. de Graffenreid, S. A. Fuqua, E. M. Curran, W. V. Welshons (1999). Loss of estrogen receptor in MCF-7 breast cancer cells is associated with reduced IGFR1 and IRS-1 expression, diminished IGF signaling, and a failure to respond mitogenically to IGFs. Proc. Endocrine Society, 81st Ann. Meeting, p. 478.
R. P. DiAugustine, P. Petrusz, G. I. Bell, C. F. Brown, K. S. Korach, J. A. McLachlan, C. T. Teng (1988). Influence of estrogens on mouse uterine epidermal growth factor precursor protein and messenger ribonucleic acid. Endocrinology 122:2355–2363.
Y. M. Huet-Hudson, C. Chakraborty, S. K. De, Y. Suzuki, G. K. Andrews, and S. K. Dey (1990). Estrogen regulates the synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol. Endocrinol. 4:510–523.
L. J. Murphy, L. C. Murphy, and H. G. Friesen (1987). Estrogen induces insulin-like growth factor-I expression in the rat uterus. Mol. Endocrinol. 1:445–450.
A. Ghahary and L. J. Murphy (1989). Uterine insulin-like growth factor-I receptors: Regulation by estrogen and variation throughout the estrous cycle. Endocrinology 125:597–604.
K. G. Nelson, T. Takahashi, N. L. Bossert, D. K. Walmer, and J. A. McLachlan (1991). Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc. Natl. Acad. Sci. U.S.A. 88:21–25.
S.W. Curtis, T. Washburn, C. Sewall, R. DiAugustine, J. Lindzey, J. F. Couse, and K. S. Korach (1996). Physiological cou pling of growth factor and steroid receptor signaling pathways: Estrogen receptor knockout mice lack estrogen-like response to epidermal growth factor. Proc. Natl. Acad. Sci. U.S.A. 93:12626–12630.
K. Rajkumar, T. Dheen, M. Krsek, and L. J. Murphy (1996). Impaired estrogen action in the uterus of insulin-like growth factor binding protein-1 transgenic mice. Endocrinology 137:1258–1264.
C. Sell, G. Dumenil, C. Deveaud, M. Miura, D. Coppola, T. DeAngelis, R. Rubin, A. Efstratiadis, R. Baserga (1994). Effect of a null mutation of the type 1 IGF receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell Biol. 14:3604–3612.
C. L. van den Berg, C. Stroh, S. G. Hilsenbeck, C-N. Weng, M. J. McDermott, G. N. Cox, and D. Yee (1997). Polyetheylene glycol conjugated insulin-like growth factor binding protein inhibits growth of breast cancer in athymic mice. Eur. J. Cancer 33:1108–1113.
J. A. Figueroa, J. Sharma, J. G. Jackson, M. J. McDermott, S. G. Hilsenbeck, D. Yee (1993). Recombinant insulin-like growth factor binding protein-1 inhibits IGF-I, serum, and estrogen-dependent growth of MCF-7 human breast cancer cells. J. Cell. Physiol. 157:229–236.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yee, D., Lee, A.V. Crosstalk Between the Insulin-Like Growth Factors and Estrogens in Breast Cancer. J Mammary Gland Biol Neoplasia 5, 107–115 (2000). https://doi.org/10.1023/A:1009575518338
Issue Date:
DOI: https://doi.org/10.1023/A:1009575518338