Abstract
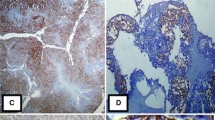
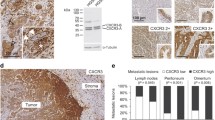
CD44 is a family of cell adhesion molecules involved in a variety of cellular functions. The present study analysed the expression of two CD44 isoforms in serous effusions of patients diagnosed with ovarian carcinoma and corresponding primary and metastatic lesions. Fifty-eight effusions, 23 primary ovarian tumours, and 44 metastatic lesions were studied for protein expression of CD44s and v3-10 using immunohistochemistry. Results were correlated with clinical parameters. CD44v3-10 was seen in carcinoma cells in the majority of cases at all sites. Malignant effusions showed an up-regulation of CD44s compared to both primary tumours and metastatic solid lesions. Mesothelial cells frequently expressed CD44s, but were rarely immunoreactive for v3-10. CD44s immunoreactivity in cancer cells in effusions was significantly more often observed in patients with FIGO stage 3 than in stage 4 patients (P = 0.045). Staining results did not correlate with age, effusion site, metastatic site, tumour grade or residual tumour mass after initial surgery. Likewise, comparison of overall and disease-free survival with expression of the CD44 isoforms studied did not reveal any statistically significant associations. The up-regulation in CD44 levels in effusions, primarily in stage 3 disease, suggests that adhesion of ovarian carcinoma cells to mesothelium may be regulated at the level of CD44s expression, and provides further evidence of phenotypic alteration in the transition from primary tumour cell clones to effusions. The similar expression profile of CD44 in carcinoma cells in peritoneal and pleural effusions supports our previous observations and the hypothesis that carcinoma cells in peritoneal effusions are truly metastatic.
Similar content being viewed by others
References
Niedbala MJ, Crickard K, Bernacki RJ. In vitro degradation of extracellular matrix by human ovarian carcinoma cells. Clin Exp Metastasis 1987; 5: 181–97.
Haynes BF, Liao HX, Patton KL. The transmembrane hyaluronate receptor (CD44): Multiple functions, multiple forms. Cancer Cells. 1991; 3: 347–50.
Ruiz P, Schwarzler C, Gunthert U. CD44 isoforms during differentiation and development. Bioessays 1995; 17: 17–24.
Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994; 76: 301–14.
Thomas L, Etoh T, Stamenkovic I et al. Migration of human melanoma cells on hyaluronate is related to CD44 expression. J Invest Dermatol 1993; 100: 115–20.
Screaton GR, Bell MV, Jackson DG et al. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 1992; 89: 12160–4.
Cannistra SA, DeFranzo B, Niloff J et al. Functional heterogeneity of CD44 molecules in ovarian cancer cell lines. Clin Cancer Res 1995; 1: 333–42.
Fox SB, Fawcett J, Jackson DG et al. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res 1994; 54: 4539–46.
Darai E, Walker-Combrouze F, Fauconnier A et al. Analysis of CD44 expression in serous and mucinous borderline tumours of the ovary: Comparison with cystadenomas and overt carcinomas. Histopathology 1998; 32: 151–9.
Uhl-Steidl M, Muller-Holzner E, Zeimet AG et al. Prognostic value of CD44 splice variant expression in ovarian cancer. Oncology 1995; 52: 400–6.
Saegusa M, Hashimura M, Machida D et al. Down-regulation of CD44 standard and variant isoforms during the development and progression of uterine cervical tumours. J Pathol 1999; 187: 173–83.
Zeimet AG, Widschwendter M, Uhl-Steidl M et al. High serum levels of soluble CD44 variant isoform v5 are associated with favourable clinical outcome in ovarian cancer. Br J Cancer 1997; 76: 1646–51.
Berner HS, Davidson B, Berner A et al. Differential expression of CD44s and CD44v3-10 in adenocarcinoma cells and reactive mesothelial cells in effusions. Virchows Arch 2000; 436: 330–5.
Bedrossian CMW. Malignant effusions: A multimodal approach to cytologic diagnosis. In Bedrossian CMW (ed): Malignant Effusions. New-York: Igaku-Shion 1994.
Davidson B, Risberg B, Kristensen G et al. Detection of cancer cells in effusions from patients diagnosed with gynaecological malignancies. Evaluation of five epithelial markers. Virchows Arch 1999; 435: 43–9.
Davidson B, Berner A, Nesland JM et al. E-cadherin and a-, b-and g-catenin protein expression is up-regulated in ovarian carcinoma cells in serous effusions. J Pathol 2000; in press.
Cannistra SA, Kansas GS, Niloff J et al. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 1993; 53: 3830–8.
Sugino T, Yoshida K, Zhao S et al. Disorderly CD44 gene expression in human cancer cells can bemodulated by growth conditions. J Pathol 1998; 186: 17–23.
Afify AM, Stern R, Joensuu H et al. Differential expression of CD44S and hyaluronic acid in malignant mesotheliomas, adenocarcinomas, and reactive mesothelial hyperplasias. Appl Immunohistochemistry 1998; 6: 11–5.
Filie AC, Abati A, Fetsch P et al. Hyaluronate binding probe and CD44 in the differential diagnosis of malignant effusions: disappointing results in cytology material [letter]. Diagn Cytopathol 1998; 18: 473–4.
Saegusa M, Machida D, Hashimura M et al. CD44 expression in benign, premalignant, and malignant ovarian neoplasms: relation to tumour development and progression. J Pathol 1999; 189: 326–37.
Kayastha S, Freedman AN, Piver MS et al. Expression of the hyaluronan receptor, CD44S, in epithelial ovarian cancer is an independent predictor of survival. Clin Cancer Res 1999; 5: 1073–6.
Rodriguez-Rodriguez L, Sancho-Torres I, Leakey P et al. CD44 splice variant expression in clear cell carcinoma of the ovary. Gynecol Oncol 1998; 71: 223–9.
Dellas A, Schultheiss E, Almendral AC et al. Expression of CD44 and variant isoforms in cervical intraepithelial neoplasia. Gynecol Oncol 1996; 62: 218–25.
Friedrichs K, Franke F, Lisboa BW et al. CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res 1995; 55: 5424–33.
Ibrahim EM, Blackett AD, Tidy JA et al. CD44 is a marker of endocervical neoplasia. Int J Gynecol Pathol 1999; 18: 101–8.
Martegani MP, Del Prete F, Gasbarri A et al. Natali PG, Bartolazzi A. Structural variability of CD44v molecules and reliability of immunodetection of CD44 isoforms using mAbs specific for CD44 variant exon products. Am J Pathol 1999; 154: 291–300.
Dall P, Heider KH, Sinn HP et al. Comparison of immunohistochemistry and RT-PCR for detection of CD44v-expression, a new prognostic factor in human breast cancer. Int J Cancer 1995; 60: 471–7.
Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol 1994; 10: 31–46.
Makar AP, Baekelandt M, Trope CG et al. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol 1995; 56: 175–80.
Puls LE, Hunter JE, Heidtman EP et al. Removal of a 130 pound ovarian neoplasm. J S C Med Assoc 1996 92: 216–9.
Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol 1993; 54: 271–335.
Lessan K, Aguiar DJ, Oegema T et al. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol 1999; 154: 1525–37.
Catterall JB, Jones LM, Turner GA. Membrane protein glycosylation and CD44 content in the adhesion of human ovarian cancer cells to hyaluronan. Clin Exp Metastasis 1999; 17: 583–91.
Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: A novel role for CD44 in the process of peritoneal implantation. Cancer Res 1997; 57: 1228–32.
Yeo TK, Nagy JA, Yeo KT et al. Increased hyaluronan at sites of attachment to mesentery by CD44-positive mouse ovarian and breast tumor cells. Am J Pathol 1996; 148: 1733–40.
Davidson B, Berner A, Risberg B et al. Carbohydrate antigen expression in primary tumors, metastatic lesions, and serous effusions from patients diagnosed with epithelial ovarian carcinoma-evidence of up-regulated Tn and Sialyl Tn antigens expression in effusions. Hum Pathol 2000; in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berner, H.S., Davidson, B., Berner, A. et al. Expression of CD44 in effusions of patients diagnosed with serous ovarian carcinoma – diagnostic and prognostic implications. Clin Exp Metastasis 18, 197–202 (2000). https://doi.org/10.1023/A:1006711320107
Issue Date:
DOI: https://doi.org/10.1023/A:1006711320107