Abstract
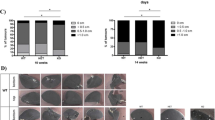
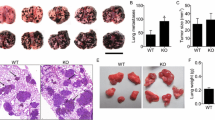
Matrix metalloproteinases (MMPs) are thought to play a key role in tumor invasion and metastasis. The role of MMP-9 (gelatinase B) in tumor metastasis was examined in MMP-9-deficient mice produced by gene targeting using embryonic stem cells. MMP-9-deficient mice develop normally and are fertile. In these mice, the number of metastatic colonies of B16-BL6 melanoma cells or Lewis lung carcinoma cells that were implanted intravenously fell by 45% for B16-BL6 melanoma and 59% for Lewis lung carcinoma (p=0.03 and p=0.0043, respectively). Gelatin zymography showed that both tumor cell lines did not secrete MMP-9 by themselves but the host cells surrounding the tumor cells secrete MMP-9 in vivo. These results indicated that host-derived MMP-9 plays an important role in the process of tumor metastasis.
Similar content being viewed by others
References
Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991; 5: 2145–2154.
Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990; 6: 121–125.
Stetler-Stevenson WG, Krutzsch HC, Wacher MP, Margulies IMK, Liotta LA. The activation of human type IV collagenase proenzyme. Biol Chem. 1989; 264: 1353–1356.
Docherty AJP, Lyons A, Smith BJ, et al. Sequence of human tissue inhibitor f metalloproteinases and its identity to erythroid-potentiating activity. Nature 1985; 318: 66–69.
Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of etalloproteinase (TIMP-2). J Biol Chem 1989 264: 17374–17378.
Liotta LA, Tryggvason K, Garbisa S et al. Metastatic potential correlates ith enzymatic degradation of basement membrane collagen. Nature 1980; 284: 67–68.
Himelstein BP, Canete-Soler R, Bernhard EJ, Dilks DW, Muschel RJ. Metalloproteinases in tumor progression: the contribution of MMP-9. Invasion & Metastasis 1995; 14: 246–258.
Stetler-Stevenson WG. Progelatinase A activation during tumor cell nvasion. Invasion & Metastasis 1995; 14: 259–268.
Sugiura Y, Shimada H, Seeger RC, Laug WE, DeClerck YA. Matrix etalloproteinases-2 and-9 are expressed in human neuroblastoma: ontribution of stromal cells to their production and correlation with metastasis. Cancer Res 1988; 58: 2209–2216.
Nielsen BS, Timshel S, Kjeldsen L et al. 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer 1996; 65: 57–62.
Segain JP, Harb J, Grégoire M, Meflah K, Menanteau J. Induction of fibroblast gelatinase B expression by direct contact with cell lines derived from primary tumor but not from metastases. Cancer Res 1996; 56: 5506–5512.
Himelstein BP, Canete-Soler R, Bernhard EJ, Muschel RJ. Induction of fibroblast 92 kDa gelatinase/type IV collagenase expression by direct contact with metastatic tumor cells. J Cell Sci 1994; 107: 477–486.
Itoh T, Ikeda T, Gomi H et al. Unaltered secretion of β-amyloid precursor protein in gelatinase A (Matrix metalloproteinase 2)-deficient mice. J Biol Chem 1997; 272: 22389–22392.
Itoh T, Tanioka M, Yoshida H et al. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 1998; 58: 1048–1051.
Yagi T, Ikawa Y, Yoshida K, et al. Homologous recombination at c-fyn locus of mouse embryonic stem cells with use of diphtheria toxin Afragment gene in negative selection. Proc Natl Acad Sci USA 1990; 87: 9918–9922.
Gomi H, Yokoyama T, Fujimoto K et al. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron 1995; 14: 29–41.
Hart IR. The selection and characterization of an invasive variant of the B16 melanoma. Am J Path 1979; 97: 587–600.
Uehira M, Matsuda H, Nakamura A, Nishimoto H. Immunologic abnormalities exhibited in IL-7 transgenic mice with dermatitis. J Invest Dermatol 1998; 110: 740–745.
Raz A, McLellan WL, Hart IR et al. Cell surface properties of B16 melanoma variants with differing metastatic potential. Cancer Res 1980; 40: 1645–1651.
Vu TH, Shipley JM, Bergers G et al. MMP-9/Gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998; 93: 411–422.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Itoh, T., Tanioka, M., Matsuda, H. et al. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin Exp Metastasis 17, 177–181 (1999). https://doi.org/10.1023/A:1006603723759
Issue Date:
DOI: https://doi.org/10.1023/A:1006603723759