Abstract
Optimizing chemotherapeutic drug delivery strategies relies, in part, on identification of the most clinically effective sequence, dose, and duration of drug exposure. The combination of dose intensive etoposide (VP‐16) followed by cyclophosphamide has clinical efficacy in the treatment of advanced breast cancer. However, molecular mechanisms that underlie the effectiveness of this combination of chemotherapeutic agents have not been investigated. In this study we investigated regulation of BAX and BCL‐2 expression by VP‐16 and cyclophosphamide as a potential mechanism for the induction of breast cancer cell death induced by this regimen.
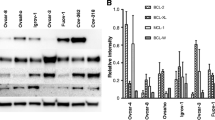
There was a dose and time dependent increase in BAX expression in the breast cancer cell lines MCF‐7, MDA‐MB‐435S, and MDA‐MB‐A231 following in vitro treatment with 50–100 μM VP‐16. Elevation of BAX protein expression in the presence of VP‐16 alone did not correlate with reduced viability or induction of apoptosis in MCF‐7, MDA‐MB‐435S, or MDA‐MB‐A231. VP‐16 did effectively block the breast cancer cell lines evaluated (MCF‐7 and MDA‐MB‐435S) at G2/M phase of the cell cycle, confirming activity of the drug in vitro. MCF‐7 and MDA‐MB‐435S cells that were pre‐treated with VP‐16 and subsequently exposed to 1.0–12.0 μg/m1 4‐hydroperoxycyclophosphamide (4HC), an active metabolite of cyclophosphamide, had markedly reduced viability when compared to matched controls treated with either VP‐16 or 4HC individually. Consistent with this loss of viability, exposure of all three cell lines to the combination of VP‐16 and 4HC resulted in higher BAX protein levels than those observed following treatment with either single agent. This combination of chemotherapeutic agents also resulted in reduced BCL‐2 expression.
These observations suggest that combination chemotherapy may derive its efficacy, in part, through coordinated regulation of specific gene products associated with apoptosis. Characterization of molecular events that underlie susceptibility of specific tumor cells to combination chemotherapeutic regimens may lead to additional improvements in treatment strategies for this disease.
Similar content being viewed by others
References
Brown RA, Herzig RH, Wolff SN, Frei-Lahr D, Pineiro L, Bolwell BJ, Harden EA, Hande KR, Herzig GP: High-dose etoposide and cyclophosphamide without bone marrow transplantation for resistant hematologic malignancy. Blood 76: 473–479, 1990
Herzig RH, Lynch J, Christiansen NP, Fay JW, Davis MP, Herzing GP: Dose-intensive chemotherapy with etoposidecylophosphamide for advanced breast cancer. Semin Oncol 23: 28–32, 1996
Barry MA, Behnke CA, Eastman A: Activation of programmed cell death (apoptosis) by cisplatin, and other anti-cancer drugs, toxins and hyperthermia. Biochem Pharmacol 40: 2353–2362, 1990
Yamada T, Chyma H: Radiation-induced interphase death of rat thymocytes is internally programmed (apoptosis). Int J Radiat Biol 53: 64–78, 1988
Alan DJ: Radiation-induced apoptosis: Its role in a MADCAT (mitosis-apoptosis differentiation-calcium toxicity) scheme of cytotoxicity mechanisms. Int J Radiat Biol 62: 145–152, 1992
Wyllie AH, Kerr JK, Currie AR: Cell death: The significance of apoptosis. Int Rev Cytol 68: 251–305, 1980
Thompson CB: Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462, 1958.
Tsujimoto Y, Gorham J, Cossman J, Jaffe B, Croce CM: The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229: 1390–1393, 1985
Simonian PL, Grillot DAM, Nunez G: Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 90: 1208–1216, 1997
Reed JC: Bcl-2 and the regulation of programmed cell death. J Cell Biol 124: 1–6, 1994
Fisher DE: Apoptosis in cancer therapy: Crossing the threshold. Cell 78: 539–542, 1994
Chen M, Quintans J, Fuks Z, Thompson C, Kufe DW, Weichselbaum RR: Suppression of Bcl-2 messenger RNA production may mediate apoptosis after ionizing radiation, tumor necrosis factor α, and ceramide. Cancer Research 55: 991–994, 1995
Simonian PL, Grillot DAM, Merino R, Nunez G: Bax can antagonize Bcl-XL during etoposide and cisplatin-induced cell death independently of its heterodimerization with Bcl-XL. J Biol Chem 37: 22764–22772, 1996
Bargou RC, Daniel PT, Mapara MY, Bommert K, Wagner C, Kallinich B, Royer HD, Doerken B: Expression of the Bcl-2 gene family in normal and malignant breast tissue: low bax-α expression in tumor cells correlates with resistance towards apoptosis. Int J Cancer 60: 854–859, 1995
Chouhei S, Sweeney EA, Shirahama T, Igarashi Y, Hakomori S, Nakatani H, Tsujimoto H, Imanishi T, Ohgaki M, Ohyama T, Yamazaki J, Hagiwara A, Yamaguchi T, Sawai K, Takahashi T: Overexpression of bax sensitizes human breast cancer MCF-7 cells to radiation-induced apoptosis. Int J Cancer 67: 101–105, 1996
Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W, Dietel M, Guski H, Geller A, Royer HD, Dorken B: Overexpression of the death-promoting gene bax-α which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest 97: 2652–2659, 1996
Wagener C, Bargou RC, Daniel PT, Bommert K, Mapara MY, Royer HD, Dorken B: Induction of the death-promoting gene Bax-α sensitizes cultured breast-cancer cells to drug-induced apoptosis. Int J Cancer 67: 138–141, 1996
Eastman A: Activation of programmed cell death by anti-cancer agents: Cisplatin as a model system. Cancer Cells 2: 275–280, 1990
Patel R, Twigg J, Crossley I, Golsteyn R, Whitaker M: Calcium-induced chromatin condensation and cyclin phosphorylation during chromatin condensation cycles in ammonia-activated sea urchin eggs. J Cell Sci S12: 129–144, 1989
Haldar S, Jena N, Croce CM: Inactivation of bcl-2 by phosphorylation. Proc Natl Acad Sci USA 92: 4507–4511, 1995
Ogretmen B, Safa AR: Down-regulation of apoptosis-related bcl-2 but not bcl-XL or bax proteins in multidrug-resistant MCF-7/Adr human breast cancer cells. Int J Cancer 67: 608–614, 1996
Fattman CL, An B, Sussman L, Dou QP: p53-independent dephosphorylation and cleavage of retinoblastoma protein during tamoxifen-induced apoptosis in human breast carcinoma cells. Cancer Lett 130: 103–113, 1998
Davidson K, Petit T, Izbicka E, Koester S, Von Hoff DD: Mitoguazone induces apoptosis via a p53-independent mechanism. Anti-cancer Drugs 9: 635–40, 1998
Gibson LF, Fortney J, Piktel D, Landreth KS, Lynch JP: Disruption of bone marrow stromal cell function by etoposide. Biology of Blood and Marrow Transplantation 3: 122–132, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gibson, L.F., Fortney, J., Magro, G. et al. Regulation of BAX and BCL‐2 expression in breast cancer cells by chemotherapy. Breast Cancer Res Treat 55, 107–117 (1999). https://doi.org/10.1023/A:1006175811676
Issue Date:
DOI: https://doi.org/10.1023/A:1006175811676