Abstract
Matrix metalloproteinases (MMPs) are a family of enzymes responsible for the breakdown of proteins of connective tissue. Through this action they play an important role in growth, development and tissue repair. Recent studies also suggest that MMPs are utilised in cancer, facilitating both local tumour invasion and metastasis. Levels of certain MMPs such as stromelysin-3 and gelatinase are elevated in tumour-associated stroma compared to non- involved tissue. A series of synthetic low molecular weight MMP inhibitors have been produced. Early inhibitors were based on the peptide structure of collagen, although more recently non-peptide inhibitors have also been developed. The inhibitors are selective for the MMP family and are active at low nanomolar concentrations. Experiments in models of breast cancer have shown that MMP inhibitors can significantly reduce the growth rate of both primary and secondary tumours, and can block the process of metastasis. Several MMP inhibitors have now started clinical trials in patients with advanced malignancy. Although not the optimum setting for a tumouristatic agent, early results suggest this approach may be effective in slowing tumour growth. Trials in the adjuvant setting will provide the most important test of these inhibitors and should determine their potential to complement existing cytoreductive treatments and prolong survival.
Similar content being viewed by others
References
Harris J, Morrow M, Norton L: Malignant tumours of the breast. In: DeVita VT, Hellman S, Rosenberg SA (eds) Cancer: Principals and Practice of Oncology, 5th Edition. Lippincott-Raven, New York, 1997, pp 1557-1616
Chu KC, Tarone RE, Kessler LG, Ries LA, Hankey BF, Miller BA, Edwards BK: Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst 88:1571-1579, 1996
Silverstein MJ, Gierson ED, Waisman JR, Senofsky GM, Colburn WJ, Gamagami P: Axillary lymph node dissection for T1a breast carcinoma. Is it indicated? Cancer 73:664-667, 1994
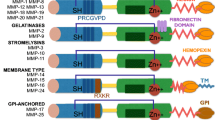
Kleiner DJ, Stetler-Stevenson WG: Structural biochemistry and activation of matrix metalloproteinases. Curr Opin Cell Biol 5:891-897, 1993
Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP: Tissue inhibitors of metalloproteinases — structure, regulation and biological functions. Eur J Cell Biol 74:111-122, 1997
Wilhelm SC, Eisen AZ, Teter M, Clark SD, Kronberger A, Goldberg G: Human fibroblast collagenase: glycosylation and tissue-specific levels of enzyme synthesis. Proc Natl Acad Sci USA 83:3756-3760, 1986
Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG: Human 92-and 72-kilodalton type IV collagenases are elastases. J Biol Chem 266:7870-7875, 1991
Shapiro SD, Kobayashi DK, Ley TJ: Cloning and characterisation of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem 268:23824-23829, 1993
Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370:61-65, 1994
Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P: A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature 348:699-704, 1990
Pei D, Majmudar G, Weiss SJ: Hydrolytic inactivation of a breast carcinoma cell-derived serpin by human stromelysin-3. J Biol Chem 269: 25849-25855, 1994
Gearing AJH, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K: Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 370:555-557, 1994
Chandler S, Cossins J, Lury J, Wells G: Macrophage metalloelastase degrades matrix and myelin proteins and processes a tumour-necrosis factor-α fusion protein. Biochem Biophys Res Commun 228:421-429, 1996
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL: A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385:729-733, 1997
Moss ML, Jin SL, Milla ME, Burkhart W, Carter HL: Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385:733-736, 1997
Beckett RP, Davidson AH, Drummond AH, Huxley P, Whittaker M: Recent advances in matrix metalloproteinase inhibitor research. Drug Dev Today 1:16-26, 1996
Brown PD, Giavazzi R: Matrix metalloproteinase inhibition: a review of anti-tumour activity. Ann Oncol 6:967-974, 1995
Grams F, Crimmin M, Hinnes L, Huxley P, Pieper M, Tschesche H, Bode W: Structure determination and analysis of human neutrophil collagenase complexed with a hydoxamate inhibitor. Biochemistry 34:14012-14020, 1995
Morphy JR, Beeley NRA, Boyce BA: Potent and selective inhibitors of gelatinase A. 2. Carboxylic acids and phosphonic acid derivatives. Bioorg Med Chem Lett 4:2747-2752, 1994
Santos O, McDermott CD, Daniels RG, Appelt K: Rodent pharmacokinetic and anti-tumor efficacy studies with a series of synthetic inhibitors of matrix metalloproteinases. Clin Exp Met 15: 499-508, 1997
Collier MA, Yuen GJ, Bansal SK, Kolis S, Chew TG, Appelt K, Clendeninn NJ: A phase I study of the matrix metalloproteinase (MMP) inhibitor AG3340 given in single doses to healthy volunteers. Proc Am Assoc Cancer Res 38:13, 1997
Lewis EJ, Bishop J, Bottomley KMK, Bradshaw D, Brewster M, Broadhurst MJ, Brown PA, Budd JM, Elliott L, Greenham AK, Johnson WH, Nixon JS, Rose F, Sutton B, Wilson K: Ro 32-3555, an orally active collagenase inhibitor, prevents cartilage breakdown in vitro and in vivo. Br J Pharmacol 121:540-546, 1997
Wood ND, Aitken M, Harris S, Kitchener S, McClelland GR, Sharp S: The tolerability and pharmacokinetics of the cartilage protective agent (Ro32-3555) in healthy male volunteers. Br J Clin Pharm 42:676-677, 1996
Beckett RP: Recent advances in the field of matrix metalloproteinase inhibitors (patent update). Exp Opin Ther Patents 6:1305-1315, 1996
Lee H-J, Chung M-C, Lee C-H, Yun B-S, Chun H-K, Kho Y-H: Gelastatins A and B, new inhibitors of gelatinase A from Westerdykella multispora F50733. J Antibiot 50:357-359, 1996
Naito K, Nakajima S, Kanbayashi N, Okuyama A, Goto M: Inhibition of metalloproteinase activity of rheumatoid arthritis synovial cells by a new inhibitor [BE16627B; L-N-(N-hydroxy-2-isobutylsuccinamoyl)-seryl-L-valine]. Agents Actions 39:182-186, 1993
Golub LM, McNamara TF, D'Angelo G, Greenwald RA, Ramamurthy NS: A non-antibacterial chemically-modified tetracycline inhibits mammalian collagenase activity. J Dental Res 66:1310-1314, 1987
Reich R, Thompson EW, Iwamoto Y, Martin GR, Deason JR, Fuller GC, Miskin R: Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res 48:3307-3312, 1988
Chirivi RS, Garofalo A, Crimmin MJ, Bawden LJ, Brown PD, Giavazzi RG: Inhibition of the metastatic spread and growth of B16–BL6 murine melanoma by a synthetic matrix metalloproteinase inhibitor. Int J Cancer 58:460-464, 1994
Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM: Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumour growth and spread in a patient-like orthotopic model in nude mice. Cancer Res 54:4726-4728, 1994
An Z, Wang X, Willmott N, Chander SK, Tickle S, Docherty AJP, Mountain A, Millican AT, Morphy R, Porter JR, Epemolu RO, Kubota T, Moossa AR, Hoffman RM: Conversion of a highly malignant colon cancer from an aggressive to a controlled disease by oral administration of a metalloproteinase inhibitor. Clin Exp Met 15: 184-195, 1997
Moses MA, Sudhalter J, Langer R: Identification of an inhibitor of neovascularization from cartilage. Science 248:1408-1410, 1990
Taraboletti G, Garofalo A, Belotti D, Drudis T, Borsotti P, Scanziani E, Brown P, Giavazzi R: Inhibition of angiogenesis and murine hemangioma growth by batimastat, a synthetic inhibitor of matrix metalloproteinases. J Natl Cancer Inst 87:293-298, 1995
Eccles SA, Box GM, Court WJ, Bone EA, Thomas W, Brown PD: Control of lymphatic and hematogenous metastases of a rat mammary carcinoma by the matrix metalloproteinase inhibitor batimastat (BB-94). Cancer Res 56:2815-2822, 1996
Sledge GW, Qulali M, Goulet R, Bone EA, Fife R: Effect of matrix metalloproteinase inhibitor batimastat on breast cancer regrowth and metastasis in athymic mice. J Natl Cancer Res 87: 1546-1550, 1995.
Galardy RE, Cassabone ME, Giese C, Gilbert JH, Lapierre F, Lopez H, Scaefer ME, Stack R, Sullivan M, Summers B, Tressler R, Tyrrell D, Wee J, Allen SD, Castellot JJ, Barletta JP, Schultz G, Fernandez LA, Fisher S, Cui T-Y, Foellmer HG, Grobelny D, Holleran WM: Low molecular weight inhibitors in corneal ulceration. Ann NY Acad Sci 732:315-323, 1994
Macaulay VM, O'Byrne KJ, Saunders MP, Salisbury A, Long L, Gleeson F, Ganesan TS, Harris AL, Talbot DC: Phase I study of matrix metalloproteinase (MMP) inhibitor batimastat (BB-94) in patients with malignant pleural effusions. Br J Cancer 71:11, 1995
Wojtowicz-Praga S, Low J, Marshall J, Ness E, Dickson R, Barter J, Sale M, McCann, P, Moore J, Cole A, Hawkins MJ: Phase I trial of a novel matrix metalloproteinase inhibitor batimastat (BB-94) in patients with advanced cancer. Invest New Drugs 14:193-202, 1996
Lauhio A, Leirisalo-Repo M, Lahdevirta J, Saikku P, Repo H: Double-blind, placebo-controlled study of the three month treatment with lymecycline in reactive arthritis, with special reference to Chlamydia arthritis. Arthritis Rheum 24:6-14, 1991
Greenwald RA, Moak SA, Golub LM: Low dose doxycycline inhibits pyridinoline excretion in selected patients with rheumatoid arthritis. Ann NY Acad Sci 732:419-421, 1994
Millar AW, Brown PD, Moore J, Galloway WA, Cornish AG, Lenehan TJ, Lynch KP: Results of single and repeat dose studies of the oral matrix metalloproteinase inhibitor marimastat in healthy male volunteers. Br J Clin Pharm, in press
Goldenberg DM, Neville A, Carter A: CEA (carcinoembryonic antigen): Its role as a marker in the management of cancer. J Cancer Res Clin Oncol 101:239-242, 1981
Steinberg W, Gelfand R, Anderson K: Comparison of the sensitivity and specificity of the CA 19-9 and CEA assays in detecting cancer of the pancreas. Gastroenterology 90:343-349, 1986
Rubin SC, Hoskins WJ, Hakes TB: CA 125 levels and surgical findings in patients undergoing secondary operations for epithelial ovarian cancer. Am J Obstet Gynecol 160:667-671, 1989
Stamey TA, Kabalin JW, McNeal JE: Prostate-specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. II. Radical prostatectomy treated patients. J Urol 141:1076-1083, 1989
Millar A, Brown P: 360 patient meta-analysis of studies of marimastat, a novel matrix metalloproteinase inhibitor. Ann Oncol 7:123, 1996
Poole C, Adams M, Barley V, Graham J, Kerr D, Louviaux I, Perren T, Piccart M, Thomas H: A dose-finding study of marimastat, an oral matrix metalloproteinase inhibitor, in patients with advanced ovarian cancer. Ann Oncol 7:68, 1996
Primrose J, Bleiberg H, Daniel F, Johnson P, Mansi J, Neoptolemos J, Seymour M, Van Belle S: A dose-finding study of marimastat, an oral matrix metalloproteinase inhibitor, in patients with advanced colorectal cancer. Ann Oncol 7:35, 1996
Parsons SL, Watson SA, Griffin NR, Steele RJC: An open phase I/II study of the oral matrix metalloproteinase inhibitor marimastat in patients with inoperable gastric cancer. Ann Oncol 7:47, 1996
Thomas H, Adams M: A phase I clinical trial of the MMP inhibitor, marimastat, administered concurrently with carboplatin to patients with relapsed ovarian cancer. Eur J Cancer 33:S246, 1997
Anderson IC, Shipp MA, Docherty AJP, Teicher BA: Combination therapy including a gelatinase inhibitor and cytotoxic agent reduces local invasion and metastasis of murine Lewis lung carcinoma. Cancer Res 56:715-718, 1996
Foekens JA, Schmitt M, van Putten WLJ, Peters HA, Bontenbal M, Janicke F, Klijn JGM: Prognostic value of urokinase-type plasminogen activator in 671 primary breast cancer patients. Cancer Res 52:6101-6105, 1992
Foekens JA, Schmitt M, van Putten WLJ, Peters HA, Kramer MD, Janicke F, Kiljn JGM: Plasminogen activator inhibitor-1 and prognosis in primary breast cancer. J Clin Oncol 12:1648-1658, 1994
Alderson MR, Hamlin I, Staunton MD: The relative significance of prognostic factors in breast carcinoma. Br J Cancer 25:646-656, 1971
Van Bogaert L-J, Maldague P: Scirrhous carcinoma of the female breast. Invest Cell Pathol 3:377-382, 1980
Anastassiades OT, Pryce PM: Fibrosis as an indication of time in infiltrating breast cancer and its importance in prognosis. Br J Cancer 29:232-239, 1974
Parham DM, Hagen N, Brown RA: Simplified method of grading primary carcinomas of the breast. J Clin Pathol 45:517-520, 1992
Carlomagno C, Perrone F, Lauria R, De Laurentiis M, Gallo C, Morabito A, Pettinato G, Panico L, Bellelli T, Apicella A, Petrella G, Bianco A, De Placido S: Prognostic significance of necrosis, elastosis, fibrosis and inflammatory cell reaction in operable breast cancer. Oncology 52: 272-277, 1995
Takei H, Iino Y, Horiguchi J, Yakoe T: Immunohistochemical fibronectin staining pattern and prognosis in invasive breast carcinoma. Oncology 52:106-111, 1995
Rights and permissions
About this article
Cite this article
Brown, P.D. Matrix metalloproteinase inhibitors. Breast Cancer Res Treat 52, 125–136 (1998). https://doi.org/10.1023/A:1006119319695
Issue Date:
DOI: https://doi.org/10.1023/A:1006119319695