-
PDF
- Split View
-
Views
-
Cite
Cite
Toru Kono, Atsushi Kaneko, Yoshiki Hira, Tatsuya Suzuki, Naoyuki Chisato, Nobuhiro Ohtake, Naoko Miura, Tsuyoshi Watanabe, Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn's disease mouse model , Journal of Crohn's and Colitis, Volume 4, Issue 2, June 2010, Pages 161–170, https://doi.org/10.1016/j.crohns.2009.09.006
Close - Share Icon Share
Abstract
Background and aims: Adrenomedullin (ADM) is a member of the calcitonin family of regulatory peptides, and is reported to have anti-inflammatory effects in animal models of Crohn's disease (CD). We investigated the therapeutic effects of daikenchuto (DKT), an extracted Japanese herbal medicine, on the regulation of endogenous ADM in the gastrointestinal tract in a CD mouse model.
Methods: Colitis was induced in mice by intrarectal instillation of 2,4,6-trinitrobenzenesulfonic acid (TNBS); afterwards, DKT was given orally. Colonic damage was assessed on day 3 by macroscopic and microscopic observation, enzyme immunoassays of proinflammatory cytokines in the colonic mucosa, and serum amyloid A (SAA), a hepatic acute-phase protein. To determine the involvement of ADM, an ADM antagonist was instilled intrarectally before DKT administration. The effect of DKT on ADM production by intestinal epithelial cells was evaluated by enzyme immunoassay and real-time PCR.
Results: DKT significantly attenuated mucosal damage and colonic inflammatory adhesions, and inhibited elevations of SAA in plasma and the proinflammatory cytokines TNFα and IFNγ in the colon. Small and large intestinal epithelial cells produced higher levels of ADM after DKT stimulation. A DKT-treated IEC-6 cell line also showed enhanced ADM production at protein and mRNA levels. Abolition of this effect by pretreatment with an ADM antagonist shows that DKT appears to exert its anti-colitis effect via up-regulation of endogenous ADM in the intestinal tract.
Conclusion: DKT exerts beneficial effects in a CD mouse model through endogenous release and production of ADM. Endogenous ADM may be a therapeutic target for CD.
1 Introduction
Adrenomedullin (ADM) is a peptide of the calcitonin family and a potent endogenous vasodilator. 1 ADM is ubiquitous in the gastrointestinal (GI) tract and plays important roles in microcirculation regulation; ADM also possesses anti-inflammatory actions via the inhibition of proinflammatory cytokines, most notably for its ability to inhibit tumor necrosis factor-α (TNFα). 2 The inhibitory effect of ADM on TNFα production has received considerable attention in the field of Crohn's disease (CD) research. Similar in many respects to therapeutic use of infliximab (which targets TNFα), ADM has advanced our understanding of possible treatment goals in CD. 3 Indeed, ADM has demonstrated anti-colitis effects in mouse 4 and rat 5 models of CD. Although the combined results of these studies suggest a novel approach to the treatment of CD with ADM, exogenous administration of ADM is clearly not practical because of the potential systemic effects of this agent and its metabolic clearance, which makes chronic delivery of small peptides impractical. 6 – 8
Daikenchuto (DKT), an extracted Japanese herbal medicine, is manufactured as a recognized prescription drug with standardized quality and ingredient quantities. 9 The formulation is composed of extract granules of Japanese pepper, processed ginger, ginseng radix and maltose powder. DKT is prescribed in Japan to improve GI motility and prevent postoperative adhesion and paralytic ileus after abdominal surgery with defined clinical efficacy. 10 – 13 In experimental studies, DKT enhanced gastrointestinal motility in vivo 14 – 16 and in vitro, 17 , 18 and prevented formation of intestinal adhesions in a rat talc-induced adhesion model. 19 , 20 We have reported that intraduodenal 21 or intracolonic 22 administration of DKT in normal rats increases small and large intestinal blood flow in a dose-dependent manner, and that this activity is abolished completely by pretreatment with the calcitonin gene-related peptide (CGRP) antagonist CGRP 8–37 . It is also known that CGRP 8–37 can block the signal pathway of ADM as well as that of CGRP, because these peptides bind to varying degrees to each other's receptors. 23 , 24 A heterodimer complex of calcitonin receptor-like receptor (CRLR) and receptor activity-modifying protein (RAMP) 1 is a known CGRP receptor, while a similar heterodimer complex of CRLR and RAMP2 or RAMP3 has been reported as the ADM receptor. ADM and CGRP, however, cross-react with one another's receptors, and thus these peptides share common biologic actions. 1 , 23 On the other hand, a decrease in endogenous CGRP is observed in animal models of CD and in clinical cases. 25 In animal models, this results in severe inflammation. 26 – 28
Based on these observations, we hypothesize that DKT-induced up-regulation of endogenous ADM may compensate for the decrease of CGRP in CD and that DKT therapy may be beneficial in the management of CD. We investigated the beneficial effects of DKT in a mouse acute colitis model using 2,4,6-trinitrobenzenesulfonic acid (TNBS), which is widely used to test potential therapeutic agents of CD. 4 , 29
2 Materials and methods
2.1 Test substances
DKT was obtained from Tsumura & Co. (Tokyo, Japan) as a water-soluble extract containing processed ginger (5.6%), ginseng radix (3.3%), Japanese pepper (2.2%), and maltose powder (88.9%). Prednisolone (PSL) was purchased from Shionogi & Co. (Osaka, Japan). The ADM antagonist ADM 22–52 was purchased from Peptide Institute Inc. (Osaka, Japan).
DKT (300, 900, or 2700 mg/10 ml/kg) was suspended in distilled water and given orally to mice at 8, 24, 32, 48, and 56 h after colitis induction. To evaluate the participation of luminal endogenous ADM, ADM 22–52 (3.57 μg/0.1 ml/mouse) was intrarectally instilled under ether anesthesia through a 3.5 F catheter inserted 3.5 cm from the anus 10 min before DKT administration in a subset of the mice. PSL (3 mg/10 ml/kg) was dissolved in distilled water, and given orally 2 and 18 h before and 8, 24, 32, 48, and 56 h after colitis induction.
2.2 Induction of acute colitis
Male BALB/c mice were purchased from Japan SLC (Shizuoka, Japan). Mice (6–8 weeks old) were anesthetized by intraperitoneal injection of sodium pentobarbital (55 mg/kg, Abbott Laboratories, North Chicago, IL) and atropine sulfate (3 mg/kg, Sigma-Aldrich, St. Louis, MO). To induce colitis, 1.5 mg TNBS (Tokyo Chemical Industry Co, Tokyo, Japan) dissolved in 0.1 ml of 50% ethanol was instilled transanally into the lumen of the colon 3.5 cm from the anus using a 3.5 F catheter. Control mice received 50% ethanol alone. Colonic damage was evaluated 3 days after the TNBS instillation. No mice died during the experiment. Ethical approval for the experimental procedures used in this study was obtained from the Asahikawa Medical College Animal Care and Use Committee. All experimental procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.3 Assessment of colonic damage
Two observers blinded to treatment group scored the colon specimens for macroscopically visible damage on a 0–10 scale according to the previously published criteria 30 with slight modification. The large intestine was removed after evaluation of the adhesion score and opened longitudinally. After washing out the luminal contents, mucosal damage in the colon was assessed macroscopically. The criteria reflect both colonic inflammatory adhesions (0 = no adhesions, 1 = minor adhesions, 2 = major adhesions) and mucosal damage described as the following: 0 = no damage, 1 = focal hyperemia with no ulcers, 2 = ulceration without hyperemia or bowel wall thickening, 3 = ulceration with inflammation at one site, 4 = two or more sites of ulceration and inflammation, 5 = two or more major sites of ulceration and inflammation or one site of ulceration extending > 1 cm along length of colon, 6–8 = damage covering > 2 cm along length of colon, with score increased by 1 for each additional centimeter of involvement. A photograph was taken to evaluate necrotic areas by image analysis (ImageJ ver.1.37 software). For histological assessment, the colon was fixed in 4% buffered paraformaldehyde. Cross-sections were stained with hematoxylin and eosin. A microscopic score was assigned on a scale of 0–11 according to previously published criteria 30 by two pathologists blinded to the experimental groups.
2.4 Determination of cytokines in colon and serum amyloid A
For determination of cytokines in the colonic mucosa, protein extracts were obtained by homogenization of the colonic mucosa (0.5 mg tissue/ml) in 50 mM Tris HCl, pH 7.4, 0.5 mM dithiothreitol, and 10 µg/ml cocktail of proteinase inhibitors containing phenylmethylsulfonyl fluoride, pepstatin, and leupeptin (Sigma-Aldrich). Samples were centrifuged at 10,000 g for 20 min at 4 °C, and the supernatants were stored at −80 °C until assay. Levels of the cytokines interleukin-1β (IL-1β), interleukin-6 (IL-6), TNFα, and interferon-γ (IFNγ) were determined by a specific sandwich ELISA using capture/biotinylated detection. Antibodies from BD Biosciences (San Jose, CA) were used according to the manufacturer's recommendations. Serum amyloid A (SAA) in plasma samples was determined using a murine ELISA kit (Tridelta Development, Morris Plains, NJ).
2.5 Immunohistochemistry for ADM
Immunohistochemistry was performed as described below. Jejunal and distal colonic tissues obtained from normal mice were fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and cut into 5-µm sections. Subsequently, serial sections were stained with rabbit polyclonal IgG antibody against ADM (Peninsula Laboratory, Inc., Belmont, CA) as the primary antibody, and peroxidase-conjugated goat anti-rabbit IgG (DAKO A/S, Glostrup, Denmark) were used as a secondary antibody. The reaction was developed by adding 3,3′ diaminobenzidine (Sigma-Aldrich) solution. All incubations were 20 min, with saturated antibody concentrations and followed by two washes.
2.6 Preparation of intestinal epithelium cells, mesenteric lymph node cells, and splenocytes
Intestinal epithelium (IE) cells were isolated according to the previously published protocol 31 with a slight modification. Briefly, the small or large bowel was removed, cut into 5 mm pieces, and washed 3 times with Hanks solution. Then, the fragments were incubated in Hanks solution (pH 7.4) containing 5 mM EDTA, 1 mM dithiothreitol, 15 mM HEPES, and 10% heat-inactivated fetal bovine serum (FBS) with continuous brisk stirring at 37 °C for 30 min. The supernatant was harvested and centrifuged at 300 g for 10 min. The pellets were suspended in Ficoll-Hypaque (Pharmacia, Piscataway, NJ), and Hanks solution containing 10% FBS was overlaid. The layer of cells at the interface after centrifugation at 710 g for 15 min was collected, washed, and applied to a 25–40% gradient of Percoll (Pharmacia). After centrifugation at 710 g for 30 min, the interface containing IE cells was collected. Following these procedures, yields of > 95% viable cells were routinely obtained. Further, purified IE cells were stained with rabbit anti-cytokeratin 12 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) following cytospin. The phenotypes of small and large intestinal IE cells were 80–90% and 95–98% cytokeratin 12 + , respectively. Twenty four hours after the culture, the cell viability was 35%, 28%, respectively.
Mesenteric lymph nodes (MLN) and spleen were removed aseptically from normal mice. Single-cell suspensions were prepared by teasing the tissues in Hanks solution containing 10% FBS. Splenocytes were prepared after being suspended in erythrocyte lysing buffer (0.155 M NH 4 Cl, 0.1 mM EDTA, and 0.01 M KHCO 3 ).
2.7 Flow cytometry analysis
Single cells were suspended in Cytofix/Cytoperm solution (BD Biosciences) for 20 min at 4 °C, washed, and then pre-incubated for 5 min at 4 °C with goat polyclonal IgG antibody (Abcam, Cambridge, UK) to reduce non-specific binding of antibodies. Next, cells were incubated for 20 min at 4 °C with rabbit polyclonal IgG antibody (4 µg/ml) against rat ADM, cytokeratin 12, or isotype control IgG (Abcam). Cells were washed, incubated for 20 min with the Alexa Fluor 488-labeled goat polyclonal antibody against rabbit IgG (Invitrogen, Carlsbad, CA), and subjected to flow cytometry analysis using a FACScalibur analyzer and CellQuest Pro software (BD Biosciences).
2.8 ADM production test
IE cells of the small or large intestine were plated in 96-well round-bottom microtiter plates at 1 × 10 6 cells/ml in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 2 mM l -glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 10 mM HEPES, and 0.1% dimethyl sulfoxide (DMSO). DKT was suspended in DMSO, diluted in DMEM, passed through a 0.45 µm filter, and then added to the cultures at final concentrations of 90, 300, or 900 µg/ml. Cells were incubated for 24 h, and ADM in the culture fluids was quantified using enzyme immunoassay (EIA) kits specific for rat ADM according to the procedure provided by the manufacturer (Phoenix Pharmaceuticals, Burlingame, CA). The rat ADM assay has 100% cross-reactivity with murine ADM. The least level of detection for ADM was 10 pg/ml.
A rat small intestine epithelial cell line, IEC-6, was obtained from Dainippon Pharmaceuticals (Osaka, Japan) and grown in DMEM supplemented with 10% FBS, 2 mM l -glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10 mM HEPES. IEC-6 cells between the 30th and 37th passage were plated in 96-well flat-bottom microtiter plates at 1 × 10 4 cells/well in DMEM supplemented with the same additives as described above, allowed to settle overnight, and then culture fluids were replaced with fresh DMEM containing 3% FBS, 0.1% DMSO, and 90, 270, or 900 µg/ml DKT passed through a 0.45 µm filter. Cells were incubated for an additional 12, 24, 48, 72, and 96 h, and ADM in the supernatants was assessed using the EIA kit.
2.9 Gene expression analysis
IEC-6 cell pellets were homogenized in QIAzol reagent (Qiagen, Valencia, CA) and total RNA was isolated using RNeasy kit (Qiagen) according to the manufacturer's recommendations. Expressions of ADM and ADM2 mRNAs were measured using a multiplex real-time quantitative RT-PCR method (TaqMan gene expression assays) and the ABI Prism 7900 sequence detection system (Applied Biosystems, Warrington, UK). Sample-to-sample variation in RNA loading was controlled by comparison with the housekeeping gene G3PDH.
2.10 Effect of ADM on proinflammatory cytokine production
MLN cells and splenocytes were cultured in 96-well flat-bottom microtiter plates pre-coated with 5 µg/ml of anti-CD3 antibody (clone 145-2C11, BD Biosciences) at 3 × 10 5 cells/well in RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mM l -glutamine, 50 µM 2-mercaptoethanol, and 10% FBS. Rat ADM (American Peptide, Sunnyvale, CA) was added at various concentrations (0.01, 0.1, or 1 µmol/L). After 24 h culture, TNFα and IFNγ concentrations in harvested culture fluids were determined as described above.
2.11 Statistics
All values are expressed as the mean ± S.E.M. The statistical significance of differences between two groups was assessed using Student's t -test. For comparisons of multiple groups, Dunnett's test was used. A probability of less than 0.05 was considered significant.
3 Results
3.1 Effect of DKT on development of acute colitis
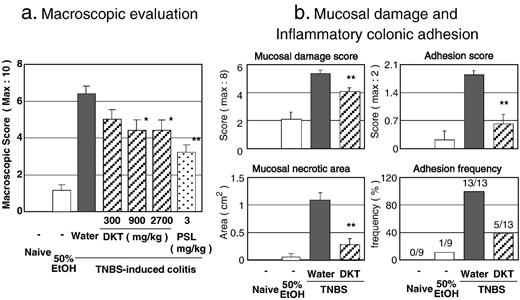
Macroscopic examination of the colons obtained 3 days after instillation of TNBS showed striking adhesion, hyperemia, inflammation, and necrosis compared with vehicle (50% EtOH)-instilled control mice. In contrast, the macroscopic evaluation scores of the colons of DKT-treated mice were lower than those of the colitis control mice in a dose-dependent manner ( Fig. 1a ). PSL was significantly effective. We performed another study to examine the effect of DKT (900 mg/kg) and to evaluate the individual results of mucosal damage and colonic inflammatory adhesion ( Fig. 1b ). DKT treatment provided significant protection against the respective parameters associated with colitis progression.
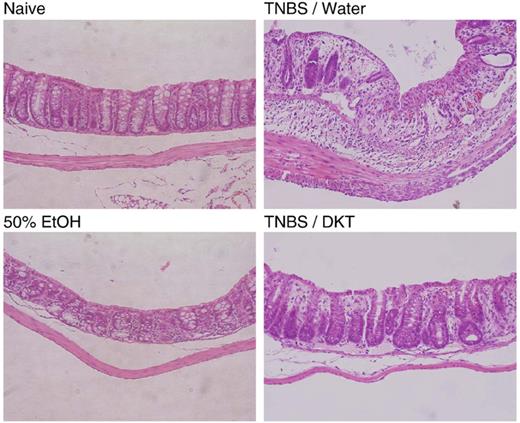
Histological examination of the colitis control specimens showed transmural inflammation with a marked increase in the thickness of the muscular layer, adherence to surrounding tissues, patchy ulceration, epithelial cell loss, pronounced depletion of mucin-producing goblet cells, reduction of the density of tubular glands, and focal loss of crypts ( Fig. 2 ). A large number of inflammatory cells infiltrated the lamina propria. The microscopic score of colitis control mice was 6.4 ± 0.1, whereas that of DKT-treated mice was 2.7 ± 0.6.
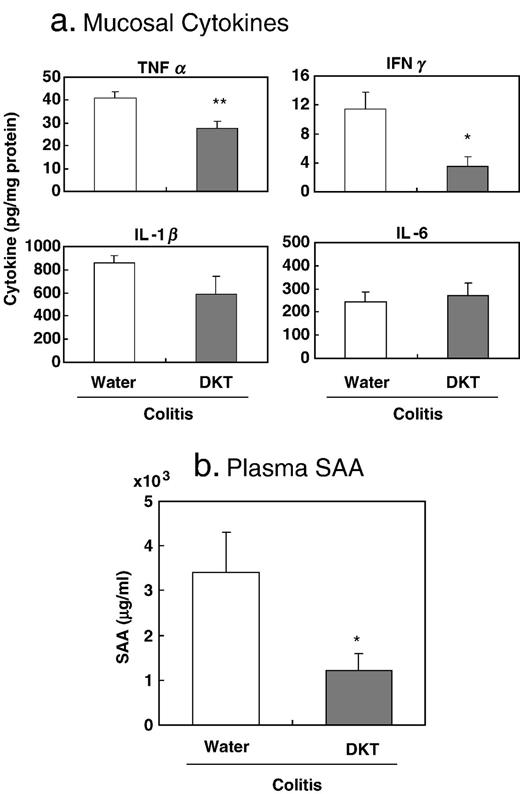
We assessed the effect of DKT (900 mg/kg) on the induction of inflammatory mediators that are mechanistically linked to colitis development. As shown in Fig. 3a , DKT treatment significantly reduced TNFα and IFNγ levels in the colonic mucosa of colitis mice compared with colitis control mice. SAA, a hepatic acute-phase protein involved in tissue damage with inflammatory conditions, was prominently increased in the plasma of colitis control mice (3398 ± 910 versus 0.5 ± 0.1 µg/ml in vehicle control mice). As shown in Fig. 3b , DKT treatment significantly decreased SAA.
To examine the involvement of luminal endogenous ADM in the anti-colitis effect of DKT, ADM 22–52 was instilled intrarectally before administration of 900 mg/kg DKT. DKT alone showed an anti-colitis effect, whereas the effect of DKT was reduced by ADM 22–52 pretreatment ( Table 1 ).
3.2 ADM enhancement by DKT in cell culture systems
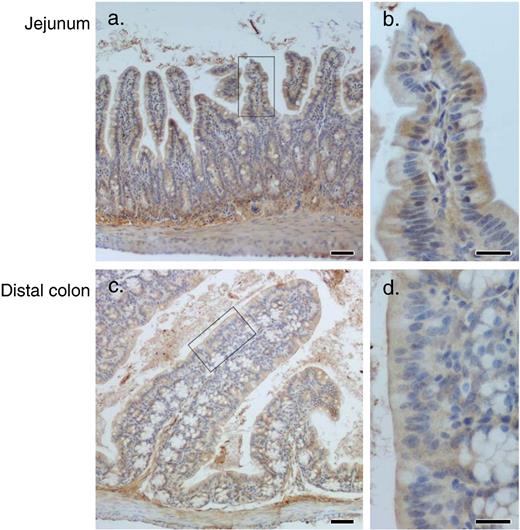
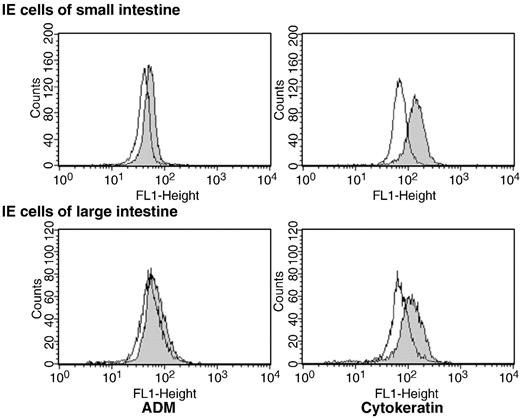
ADM immunoreactivity was mainly observed on the apical side of intestinal mucosa surface columnar epithelia ( Fig. 4 ). In addition, ADM immunoreactivity was observed in the subepithelial sites. To investigate the possibility that DKT affects release of ADM from the IE, IE cells were isolated from the small and large intestines. First, phenotypic analysis was performed using flow cytometric techniques. As shown in Fig. 5 , IE cells of both small and large intestines expressed cytokeratin and ADM. Next, an ADM production test was performed. As shown in Table 2 , 900 µg/ml DKT enhanced ADM production in both small and large intestinal IE cells.
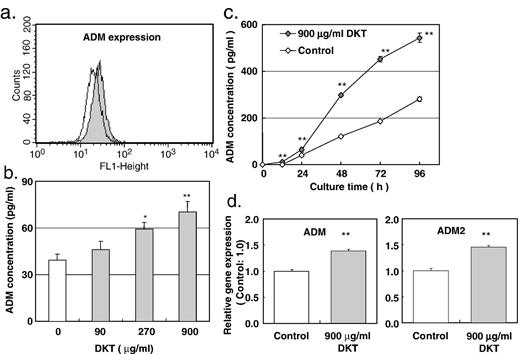
Because primary cultured IE cells do not proliferate during the ADM production test, a cell line of rat small intestinal epithelial cell IEC-6 was utilized to further examine the ADM-enhancing activity of DKT. As indicated by flow cytometric analysis, IEC-6 cells expressed intracellular ADM ( Fig. 6a ) and DKT enhanced ADM production in a concentration-dependent manner ( Fig. 6b ). Addition of 900 µg/ml DKT to culture fluids of IEC-6 cells resulted in an ADM concentration of 70 ± 7 pg/ml, significantly higher than that of the control (39 ± 4 pg/ml). Moreover, ADM production by IEC-6 cells treated with DKT (900 µg/ml) was higher than that of the control at any time point ( Fig. 6c ). Examination of the mRNA expression levels of ADM and ADM2 in IEC-6 cells treated with or without DKT (900 µg/ml) by real-time PCR revealed that DKT significantly up-regulated both ADM and ADM2 gene expression.
3.3 Inhibitory effect of ADM on cytokine production by MLN cells and splenocytes
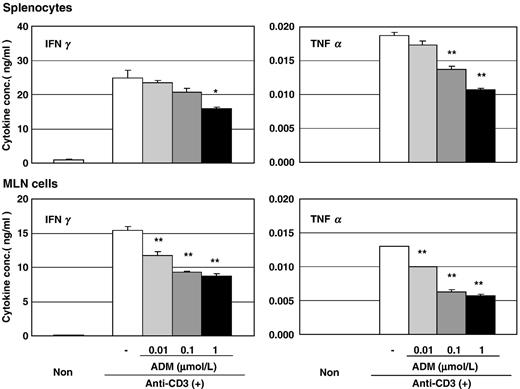
To test the anti-inflammatory effect of ADM, MLN cells and splenocytes were cultured in plates coated with anti-CD3 in the presence of various concentrations of ADM. TNFα and IFNγ were determined in this study as representative proinflammatory cytokines related to enteritis. As shown in Fig. 7 , both cytokines were significantly decreased in ADM-treated cells compared with untreated control.
4 Discussion
In this study, ADM immunoreactivity was abundant in intestinal epithelial mucosa. Several investigators have suggested that ADM plays an important role in mucosal defense as an antimicrobial peptide. 32 , 33 Invasion of microbes through the mucosal barrier stimulates the host immune system and intimately correlates with the development of morbidity in experimental and human inflammatory bowel disease. 34 Besides epithelial cells, ADM is known to be synthesized and secreted from vascular smooth muscle cells, endothelial cells, fibroblasts, neuronal cells, and immune cells, 35 however, we did not identify the ADM-staining cells in subepithelial sites.
It has already been verified that ADM diminishes proinflammatory cytokine production. 36 Thus, ADM may play a regulatory role in inflammatory gut diseases such as CD. Actually, the anti-colitis effect of ADM was previously demonstrated in mouse 4 and rat 5 models of CD. Combined results of these studies support the potential treatment of CD with ADM, as exogenous ADM administration has proven efficacy in animal models.
However, clinical application of exogenous ADM as a therapeutic agent is clearly impossible because of its effects on the entire systemic circulation, and rapid metabolic clearance makes delivery impractical. 6 – 8 Thus, it is more desirable to develop an agent that causes release and production of endogenous ADM in the bowel rather than supplying exogenous ADM. As shown in Table 2 and Fig. 6 , DKT stimulates and enhances ADM production by IE cells and the IEC-6 cell line. Messenger RNA expressions of ADM and ADM2 were prominently increased by DKT stimulation. ADM2 is a peptide with 33% sequence homology to ADM, binding to ADM and CGRP receptors. 37 Indeed, ADM2 has many biological effects similar to those of ADM and CGRP, and could be important for regulation of diverse physiological processes that have been attributed to CGRP and ADM. Several investigators demonstrated that various cells produce ADM and ADM2 and that their production profiles are modified by inflammation-related substances including cytokines and steroid hormones. 35 , 38
The main ingredients of DKT have been identified by three-dimensional high-performance liquid chromatography. 14 A study of the active ingredients that enhance ADM production is now being performed. According to our preliminary tests, 6-shogaol and hydroxy α-sanshool, the primary ingredients of DKT, have the ability to enhance ADM synthesis in an ADM production test using IEC-6 cells. It is of interest that these ingredients elevate intestinal blood flow when they are administered to the intestinal tract. 21 , 22 In the current study, we investigated the relationship between the anti-colitis effect of DKT and endogenous ADM in the bowel using ADM 22–52 . Pretreatment with ADM 22–52 reduced the anti-colitis effect of DKT. Moreover, we demonstrated that DKT induces the release of ADM from IE cells in a dose-dependent manner. These lines of evidence indicate that endogenous epithelial ADM is directly up-regulated by luminal DKT. Further studies are needed to elucidate the precise mechanisms underlying up-regulation of endogenous ADM in the intestine.
We confirmed that DKT increased the blood flow at ischemia sites found in chronically inflamed colons in a TNBS-induced rat colitis model (data not shown), as well as in a normal rat model. 21 , 22 Recurrent strictures frequently emerge around anastomosis sites in CD. This morbidity is related to local ischemia at the anastomosis site, although its precise mechanism is not clear. It is speculated that recurrent lesions at an anastomosis site in clinical practice may be reduced by postoperative DKT administration.
The inhibitory effect of ADM on TNFα and IFNγ production has been reported previously. 5 , 36 As shown in Fig. 3a , administration of DKT, an enhancer of endogenous ADM, resulted in reduction of TNFα and IFNγ levels in the colonic mucosa of colitis mice. DKT-induced inhibition of TNFα and IFNγ production has received considerable attention in the field of CD, similar in many respects to the advances in CD treatment with infliximab, which targets TNFα. 3 Recent study revealed that there is a potential advantage of targeting IFNγ for the treatment of CD. Neutralization of IFNγ may interrupt the cytokine cascade that leads to TNFα up-regulation, resulting in decreased TNFα and IFNγ levels. 39 In fact, some of the clinical effects of anti-IFNγ therapy have been reported. 40 , 41 Based on these facts, targeting endogenous ADM may be a potential advantage for the CD treatment. Additionally, DKT may be a unique therapeutic agent for CD as an intestinal endogenous ADM enhancer.
Finally, as shown in Fig. 1b , DKT dramatically inhibited formation of inflammatory adhesions between the inflamed colon and adjacent tissue. Bowel adhesions are frequently found in CD, associated with morbidity in the form of obstruction and fistula formation, and may require surgical removal of the bowel. Recent studies have confirmed that IFNγ plays a crucial role in the regulation of fibrous tissue formation by disrupting the balance between plasminogen activator inhibitor type 1 and tissue-type plasminogen activator, which reciprocally regulate fibrin deposition. 42 It is plausible that the anti-adhesion effect of DKT is due to the reduction in IFNγ, which can be down-regulated by ADM; however, a precise mechanism of the anti-adhesion effect of ADM is still unclear. Interestingly, for nearly a decade in Japan, DKT has been employed to speed the recovery from postoperative ileus after abdominal surgery and its efficacy has been reported in clinical 11 and animal study. 19 The Japanese government has covered DKT as a prescription drug under their national health insurance since 1986. In addition, DKT has been on the market in Japan for several decades and is associated with very few side effects. 43
In summary, our findings indicate that DKT attenuates mucosal damage, colonic inflammatory adhesions, systemic inflammation, and inhibited mucosal proinflammatory cytokines, including TNFα and IFNγ, in a CD mouse model via up-regulation of endogenous ADM in the IE. Endogenous ADM in epithelial cells may be a unique therapeutic target for CD morbidity.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank Drs. Yuji Omiya, Masahiro Yamamoto, Shinichi Kasai, and Yoshio Kase for the helpful discussions and Akihito Mase for the technical assistance.
References
Figures
Protective effect of DKT on 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis model. Macroscopically visible damage was evaluated 3 days after TNBS instillation. (a) Colitis was scored on a 0–10 scale, which was a sum of the mucosal damage score (0–8) and the colonic adhesion score (0–2). DKT (300, 900, or 2700 mg/kg) was given orally 8, 24, 32, 48, and 56 h after TNBS instillation. N = 9. (b) DKT was given orally at 900 mg/kg. Clinical severity was monitored by mucosal damage score, necrotic area of colonic mucosa, adhesion score, and adhesion frequency. N = 9 (naive and 50% EtOH), 13 (colitis groups). *, **: P < 0.05, 0.01 versus TNBS/water (colitis control), respectively.
Effect of DKT on histological evaluation in colitis mice. DKT was given orally at 900 mg/kg after 2,4,6-trinitrobenzenesulfonic acid (TNBS) instillation. Three days after TNBS instillation, histopathological analysis (×40) of hematoxylin and eosin-stained sections of the colon was performed.
Effects of DKT on the mucosal and systemic inflammatory responses in colitis mice. Three days after colitis induction, colonic mucosa and plasma were collected. (a) Concentrations of cytokines in protein extracts of the colonic mucosa were determined by ELISA, N = 6. (b) Serum amyloid A (SAA) concentration in plasma was determined by ELISA. N = 11. SAA of naive and vehicle (50% EtOH)-treated control mice were less than 1 µg/ml, respectively. *, **: P < 0.05, 0.01 versus colitis control, respectively.
Expression of adrenomedullin (ADM) in mucosal epithelium of intestinal tract. Jejunum and distal colon were obtained from normal mice, and stained with rabbit anti-ADM antibody. Scale bar shown in panels a and c is 50 µm; scale bar shown in panels b and d is 20 µm.
Phenotypic characterization of IE cells from small and large intestines. IE cells isolated from small and large intestines of normal mice were enriched using density gradient centrifugation and analyzed by flow cytometric techniques. Adrenomedullin (ADM) and cytokeratin were stained with the respective specific antibody (grey histograms) after cells were suspended in Cytofix/Cytoperm solution. Isotype controls are shown as open histograms.
DKT-enhanced ADM production by IEC-6 cell line. (a) A line of rat small intestine epithelial cells, IEC-6, was stained with anti-adrenomedullin (ADM) polyclonal antibody, and determined by flow cytometry. (b) IEC-6 cells were plated in 96-well flat-bottom plates at 1 × 10 4 cells/well. The next day, culture fluids were replaced with fresh medium containing DKT (90, 270, or 900 µg/ml). Cells were allowed to incubate an additional 24 h, and the supernatants were harvested. Concentrations (pg/ml) of adrenomedullin (ADM) in culture fluids were determined using the EIA method. N = 3. (c) A time-course study was performed. IEC-6 cell was cultured with or without 900 µg/ml of DKT for 12, 24, 48, 72, or 96 h. N = 3. (d) Total RNA was isolated from IEC-6 cell cultured with or without 900 µg/ml of DKT for 24 h. Expressions of ADM and ADM2 mRNA were measured by real-time PCR and normalized by GAPDH expression. N = 5. *, **: P < 0.05, 0.01 versus no DKT control, respectively.
Inhibitory effect of ADM on TNFα and IFNγ production by immune cells. Mesenteric lymph node (MLN) cells and splenocytes were isolated from normal mice and cultured at 3 × 10 5 cells/well for 24 h in 96-well flat-bottom microtiter plates pre-coated with anti-CD3 antibody. Adrenomedullin (ADM) was added at final concentrations of 0.01, 0.1, or 1 µmol/l. Concentrations (pg/ml) of TNFα and IFNγ in culture fluids were determined by ELISA. N = 3. *, **: P < 0.05, 0.01 versus no ADM control, respectively.
Tables
Abolition of anti-colitis effect of DKT by pretreatment with ADM antagonist.
| Induction | Administration | N | Macroscopic score | |
| Oral | Intrarectal | (Max: 10) | ||
| Experiment 1 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 0.50 ± 0.34 |
| TNBS | Water | – | 8 | 6.13 ± 0.23 |
| TNBS | DKT | – | 8 | 4.25 ± 0.59 a |
| TNBS | Water | Saline | 8 | 5.38 ± 0.46 |
| TNBS | Water | ADM 22–52 | 8 | 6.00 ± 0.42 |
| TNBS | DKT | ADM 22–52 | 8 | 5.50 ± 0.50 NS |
| Experiment 2 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 1.67 ± 0.49 |
| TNBS | Water | Saline | 8 | 7.13 ± 0.52 |
| TNBS | DKT | Saline | 8 | 5.25 ± 0.45 b |
| TNBS | Water | ADM 22–52 | 8 | 6.88 ± 0.35 |
| TNBS | DKT | ADM 22–52 | 8 | 6.63 ± 0.53 NS |
| Induction | Administration | N | Macroscopic score | |
| Oral | Intrarectal | (Max: 10) | ||
| Experiment 1 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 0.50 ± 0.34 |
| TNBS | Water | – | 8 | 6.13 ± 0.23 |
| TNBS | DKT | – | 8 | 4.25 ± 0.59 a |
| TNBS | Water | Saline | 8 | 5.38 ± 0.46 |
| TNBS | Water | ADM 22–52 | 8 | 6.00 ± 0.42 |
| TNBS | DKT | ADM 22–52 | 8 | 5.50 ± 0.50 NS |
| Experiment 2 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 1.67 ± 0.49 |
| TNBS | Water | Saline | 8 | 7.13 ± 0.52 |
| TNBS | DKT | Saline | 8 | 5.25 ± 0.45 b |
| TNBS | Water | ADM 22–52 | 8 | 6.88 ± 0.35 |
| TNBS | DKT | ADM 22–52 | 8 | 6.63 ± 0.53 NS |
DKT was given orally at doses of 900 mg/kg after 2,4,6-trinitrobenzene sulfonic acid (TNBS) instillation. An adrenomedullin (ADM) antagonist (ADM 22–52 , 3.57 μg/0.1 ml/mouse) was instilled intrarectally 10 min before DKT administration. Macroscopic evaluation was performed 3 days after colitis induction. Significant analysis was performed by Student's t -test, Water versus DKT. a,b : P < 0.05, NS: Not significant.
Abolition of anti-colitis effect of DKT by pretreatment with ADM antagonist.
| Induction | Administration | N | Macroscopic score | |
| Oral | Intrarectal | (Max: 10) | ||
| Experiment 1 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 0.50 ± 0.34 |
| TNBS | Water | – | 8 | 6.13 ± 0.23 |
| TNBS | DKT | – | 8 | 4.25 ± 0.59 a |
| TNBS | Water | Saline | 8 | 5.38 ± 0.46 |
| TNBS | Water | ADM 22–52 | 8 | 6.00 ± 0.42 |
| TNBS | DKT | ADM 22–52 | 8 | 5.50 ± 0.50 NS |
| Experiment 2 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 1.67 ± 0.49 |
| TNBS | Water | Saline | 8 | 7.13 ± 0.52 |
| TNBS | DKT | Saline | 8 | 5.25 ± 0.45 b |
| TNBS | Water | ADM 22–52 | 8 | 6.88 ± 0.35 |
| TNBS | DKT | ADM 22–52 | 8 | 6.63 ± 0.53 NS |
| Induction | Administration | N | Macroscopic score | |
| Oral | Intrarectal | (Max: 10) | ||
| Experiment 1 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 0.50 ± 0.34 |
| TNBS | Water | – | 8 | 6.13 ± 0.23 |
| TNBS | DKT | – | 8 | 4.25 ± 0.59 a |
| TNBS | Water | Saline | 8 | 5.38 ± 0.46 |
| TNBS | Water | ADM 22–52 | 8 | 6.00 ± 0.42 |
| TNBS | DKT | ADM 22–52 | 8 | 5.50 ± 0.50 NS |
| Experiment 2 | ||||
| – | – | – | 6 | 0.00 ± 0.00 |
| 50% EtOH | – | – | 6 | 1.67 ± 0.49 |
| TNBS | Water | Saline | 8 | 7.13 ± 0.52 |
| TNBS | DKT | Saline | 8 | 5.25 ± 0.45 b |
| TNBS | Water | ADM 22–52 | 8 | 6.88 ± 0.35 |
| TNBS | DKT | ADM 22–52 | 8 | 6.63 ± 0.53 NS |
DKT was given orally at doses of 900 mg/kg after 2,4,6-trinitrobenzene sulfonic acid (TNBS) instillation. An adrenomedullin (ADM) antagonist (ADM 22–52 , 3.57 μg/0.1 ml/mouse) was instilled intrarectally 10 min before DKT administration. Macroscopic evaluation was performed 3 days after colitis induction. Significant analysis was performed by Student's t -test, Water versus DKT. a,b : P < 0.05, NS: Not significant.
Increase of ADM production in murine intestinal epithelial cells by DKT.
| Cells | DKT concentration (µg/ml) | |||
| 0 | 90 | 270 | 900 | |
| IE cells of small intestine | 31 ± 4 | 38 ± 7 | 46 ± 5 | 54 ± 2 a |
| IE cells of large intestine | 3 ± 1 | 13 ± 1 b | 11 ± 3 b | 11 ± 1 b |
| Cells | DKT concentration (µg/ml) | |||
| 0 | 90 | 270 | 900 | |
| IE cells of small intestine | 31 ± 4 | 38 ± 7 | 46 ± 5 | 54 ± 2 a |
| IE cells of large intestine | 3 ± 1 | 13 ± 1 b | 11 ± 3 b | 11 ± 1 b |
Intestinal epithelial (IE) cells were isolated from the small or large intestine of normal mice and stimulated with DKT (90, 270, or 900 µg/ml) at 1 × 10 6 cells/ml in 96-well round-bottom plates for 24 h. Concentrations (pg/ml) of adrenomedullin (ADM) in culture fluids were determined using the EIA method. N = 4–6, a, b : P < 0.05, 0.01 versus no DKT control, respectively.
Increase of ADM production in murine intestinal epithelial cells by DKT.
| Cells | DKT concentration (µg/ml) | |||
| 0 | 90 | 270 | 900 | |
| IE cells of small intestine | 31 ± 4 | 38 ± 7 | 46 ± 5 | 54 ± 2 a |
| IE cells of large intestine | 3 ± 1 | 13 ± 1 b | 11 ± 3 b | 11 ± 1 b |
| Cells | DKT concentration (µg/ml) | |||
| 0 | 90 | 270 | 900 | |
| IE cells of small intestine | 31 ± 4 | 38 ± 7 | 46 ± 5 | 54 ± 2 a |
| IE cells of large intestine | 3 ± 1 | 13 ± 1 b | 11 ± 3 b | 11 ± 1 b |
Intestinal epithelial (IE) cells were isolated from the small or large intestine of normal mice and stimulated with DKT (90, 270, or 900 µg/ml) at 1 × 10 6 cells/ml in 96-well round-bottom plates for 24 h. Concentrations (pg/ml) of adrenomedullin (ADM) in culture fluids were determined using the EIA method. N = 4–6, a, b : P < 0.05, 0.01 versus no DKT control, respectively.
Author notes
Part of this work was presented at Digestive Disease Week 2009, American Gastroenterologic Association (AGA), Chicago, USA.