Abstract
This study aims to evaluate the relationship between the cyclooxygenase 2 (COX2) G1195A (rs689465) polymorphism and the risk of prostate cancer in a Japanese population and the associations between COX2 polymorphisms and clinicopathological characteristics, including Gleason grade and prostate-specific antigen (PSA) grade. We recruited 134 patients with prostate cancer and 86 healthy controls matched for age and smoking status. The COX2 G1195A polymorphism status was determined by polymerase chain reaction and restriction fragment length polymorphism analysis. Genotype distributions (p = 0.028) and allelic frequencies (p = 0.014) differed significantly between prostate cancer and control groups in terms of the COX2 G1195A polymorphism (Pearson’s χ 2 test). Logistic regression analysis of case and control outcomes showed an odds ratio between the GG and AA genotypes of 3.15 (95 % confidence interval = 1.27–8.08, p = 0.014), indicating an increased risk of prostate cancer associated with the AA genotype. Subset analysis revealed no significant associations between this polymorphism and clinicopathological characteristics of prostate cancer. This study demonstrated a relationship between the COX2 G1195A variant and prostate cancer risk. This polymorphism may merit further investigation as a potential genomic marker for the early detection of prostate cancer. Our results support the hypothesis that rs689465 influences susceptibility to prostate cancer; however, prostate cancer progression was not associated with rs689465 in a Japanese population.
Similar content being viewed by others
Prostate cancer is one of the most common types of cancer and is the sixth leading cause of cancer-related deaths among Japanese men [1]. It is now a serious public health issue in Japan, with the death rate increasing over the past two decades [1]. In most cases, death from prostate cancer results from metastatic disease. Understanding the mechanisms underlying its progression will facilitate the development of biomarkers and novel therapeutic strategies to control this devastating malignancy.
A strong association exists between states of chronic inflammation and cancer, and it is believed that mediators of inflammation may be responsible for this phenomenon [2]. Chronic inflammation can lead to carcinogenesis by damaging DNA through radical oxygen and nitrogen species, enhancing cell proliferation, and stimulating angiogenesis [3]. Moreover, some single nucleotide polymorphisms (SNPs) in cytokine genes have been proven to influence protein expression and/or activity, which may predispose affected individuals to develop particular cancers [4–6].
Cyclooxygenase (COX) is involved in several inflammatory pathways, and the two COX isoforms, COX1 and COX2, differ both in their regulation and tissue distribution. COX2, or prostaglandin-endoperoxide synthase 2 (PTGS2), is an enzyme inducible by cytokines, among other factors, that catalyzes the conversion of arachidonic acid to prostaglandins in response to inflammatory stimuli [7]. It is also involved in ovulation. Prostaglandins may be involved in the pathogenesis of cancers and are induced in many malignant tissues through overexpression of COX2 [8], which also cause hyperproliferation, transformation, tumor growth, invasion, and metastasis, thus potentially increasing the risk of prostate cancer. Several studies showed that COX2 is overexpressed in prostate cancer tissue compared with benign tissue from the same patient, while in vitro COX2 overexpression inhibits apoptosis and induces tumor angiogenesis [9].
The association between COX2 SNPs and prostate cancer susceptibility has previously been examined in Western [10–12] and African populations [13], but never in Japanese individuals. The COX2 gene on chromosome 1q25.2–q25.3 is a candidate gene for prostate cancer susceptibility because of its proximity to the first mapped locus for this on chromosome 1q24–q25 (hereditary prostate cancer 1, HPC1), recognized in a 1996 genetic linkage study [14].
Emerging evidence for a role for COX2 in carcinogenesis prompted us to investigate the relationship between different alleles of this gene and prostate cancer. We therefore aimed to determine the genotypic frequency of the COX2 G1195A polymorphism and its association with prostate cancer susceptibility. We also analyzed the relationship between COX2 polymorphisms and clinicopathological characteristics, such as the Gleason grade and prostate-specific antigen (PSA) grade. To the best of our knowledge, this is the first study to evaluate the contribution of COX2 polymorphisms to prostate oncology in a Japanese population.
Materials and methods
Study participants
The study consisted of a total of 220 Japanese men, including 134 histologically confirmed cases of prostate cancer and 86 healthy age-, ethnicity-, and smoking status-matched controls. The patients with prostate cancer were treated at the Department of Urology, Miyazaki Medical University Hospital (Miyazaki, Japan) and its related hospitals between August 2011 and October 2012. Tumor grade was evaluated using the Gleason scoring system. Controls were selected randomly from healthy individuals with no history of prostate cancer or any other cancers. All controls received a PSA test to detect occult prostate cancer. All participants were informed of the details, procedures, and objectives of this study. During the study period, critical information such as age and smoking status was collected from the participants using a standardized questionnaire. This study was approved by the Ethics Committee of Miyazaki Medical University and related hospitals.
Assessment of smoking status
Information on demographics, smoking history, family history of cancer, and medical history was collected during the interview. Participants were asked about their smoking status and were classified as “smokers” or “nonsmokers.” Those who had never smoked or had smoked only a few packs of cigarettes during their lifetime were defined as nonsmokers. Those who had smoked cigarettes regularly for more than 20 years were defined as smokers. Both cases and controls were subjected to similar protocols/questionnaires by the same interviewer.
COX2 genotyping
Genomic DNA was extracted from peripheral blood leukocytes using the DNA Extractor WB Kit (Wako Pure Chemical Industries Ltd., Osaka, Japan) according to the manufacturer’s instructions and eluted with 100 μl TE buffer (Nacalai Tesque, Tokyo, Japan). COX2 G1195A genotypes were determined using the polymerase chain reaction (PCR)-based restriction fragment length polymorphism assay, as described previously [15, 16]. The following primers were used: COX2 G1195A, forward, 5'-CCCTGAGCACTACCCATGAT-3′, and reverse, 5′-GCCCTTCATAGGAGATACTGG-3′. Reactions were performed using KAPA Taq PCR Kits (Nippon Genetics, Tokyo, Japan) in a thermal cycler (TaKaRa PCR Thermal Cycler Dice; Takara, Tokyo, Japan).
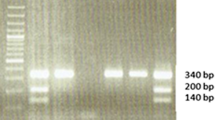
PCR conditions consisted of 1 cycle at 95 °C for 5 min and then 40 cycles of 95 °C for 30 s, 61 °C for 40 s, and 72 °C for 45 s, with a final extension at 72 °C for 10 min. The PCR products were digested overnight with PvuII restriction enzyme (Takara, Tokyo, Japan) at 37 °C, and the fragments were separated on a 3 % agarose gel. A 273-bp band corresponded to COX2 AA; 273-, 220-, and 53-bp bands represented heterozygous individuals, and bands of 220- and 53-bp corresponded to the homozygous GG genotype (Fig. 1).
Statistical analysis
Statistical analysis was performed using the R i386 2.15.1 software package (Vienna University of Economics and Business Administration, Vienna, Austria). The significance of differences in COX2 G1195A genotypes between cases and controls was determined by Pearson’s χ 2 tests. Probability values of <0.05 were regarded as statistically significant. Odds ratios (ORs) and 95 % confidence intervals (CIs) for prostate cancer were calculated by multivariate logistic regression analysis after adjusting for several confounding variables such as age and smoking status.
Results
Clinicopathological features are shown in Table 1. The mean age of cases and controls was 68.3 ± 7.4 (range, 61–75) years and 66.9 ± 8.3 (range, 59–75) years, respectively. There were no significant differences between prostate cancer cases and controls in terms of mean age distribution (p = 0.21, not significant (NS)) or relative frequencies of smokers and nonsmokers (p = 0.24, NS).
COX2 G1195A genotypic and allelic frequencies are shown in Table 2. The genotype and allele frequencies were in Hardy–Weinberg equilibrium. Genotypic distributions (p = 0.028) and allelic frequencies (p = 0.014) differed significantly between prostate cancer and control groups in terms of the COX2 G1195A polymorphisms. The frequency of A allele carriers was higher in cases than in controls. Logistic regression analysis of outcomes (adjusted for age at diagnosis and smoking status) showed that the AA genotype was associated with increased susceptibility to prostate cancer (OR = 3.15, 95 % CI = 1.27–8.08, p = 0.014). Subset analysis to investigate possible associations between COX2 polymorphisms and clinicopathological characteristics such as Gleason grade and PSA grade revealed no significant associations.
COX2 genotype and risk associated with Gleason grade
COX2 genotypes were further analyzed for a risk associated with less aggressive or highly aggressive disease, based on Gleason grade. Patients were then categorized into three groups based on this combined score (Gleason score of ≤6, low grade; Gleason score 7, intermediate grade; Gleason score of ≥8, high grade). The results demonstrated no significant associations between genotype and Gleason grade (Table 3).
COX2 genotype and risk associated with PSA grade
Patients were categorized into three groups based on PSA value (PSA of <10.0 ng/ml, low grade; PSA of ≥10 and <20.0 ng/ml, intermediate grade; PSA of ≥20.0 ng/ml, high grade). No significant associations were found between COX2 polymorphisms and PSA grade in patients with prostate cancer (Table 3).
Discussion
In recent years, interest in cancer genetic susceptibility has increased, with attention being focused on the study of SNPs in genes associated in carcinogenesis as they may contribute to individual susceptibility to cancer [17]. We consider COX2 located in the HPC1 region 1q25 [14] to be of particular interest in the search for prostate cancer susceptibility genes. COX2 is inducible by a variety of factors such as cytokines, growth factors, and tumor promoters and is highly expressed in several human cancers and cancer cell lines, including prostate cancer [18].
Associations of COX2 polymorphisms with prostate cancer indicate that inflammation is an important factor in the development of this cancer. However, to date, these SNPs have not been extensively explored. We selected the COX2 G1195A polymorphism from the National Center for Biotechnology Information website and found a significant association of the A variant with higher susceptibility for prostate cancer in our Japanese population.
Similar results were previously reported by Zhang et al. and Gao et al. [19, 20], who showed that the G1195A A variant was significantly associated with esophageal squamous cell carcinoma and breast cancer, respectively, in Chinese populations. Similarly, in a case–control study of 377 oral squamous cell carcinoma (OSCC) patients and 442 controls from Taiwan, AA homozygotes were shown to have a potential genetic risk of developing OSCC. The strongest risk was observed in AA homozygote betel chewers, while AG and AA genotype non-betel chewers who smoked and consumed alcohol had increased risks of 15.1- and 32.1-fold, respectively [21]. However, the GA and AA genotypes showed no significant association with head and neck cancer risk in a Dutch population [22]. This could be explained by the fact that COX2 polymorphisms vary in different tumors and between different ethnic groups. Moreover, the presence of COX2 GA or AA polymorphisms was reported to create a c-myb binding site and to enhance transcriptional activity of the COX2 gene [19].
Recently, several studies demonstrated that COX2 variants were associated with the risk of prostate cancer [11, 12, 23, 24]. In a Swedish population, two COX2 variants, C3100T (rs689470) and C8365T (rs2043), were associated with a reduced risk of prostate cancer [12]. Another study focusing on advanced prostate cancer patients in African-Americans and European Americans showed that three of the nine SNPs examined demonstrated significant associations with prostate cancer risk, with the most compelling polymorphism, rs2745557, associated with a lower risk of disease [11]. Finally, Wu et al. reported that the G allele of COX2 promoter G765C appears to be associated with prostate cancer development and may be a useful biomarker for its early detection [23].
In summary, our results are the first to indicate that prostate cancer susceptibility and risk are influenced by a common COX2 genetic polymorphism, with the COX2 G1195A A allele shown to increase the risk of developing prostate cancer. They are also the first to suggest that the COX2 G1195A polymorphism plays an important role in prostate cancer susceptibility in the Japanese population. However, we found no significant associations between this polymorphism and clinicopathological characteristics associated with prostate cancer progression. This apparent discrepancy could be attributed to the small number of cases (n = 134) in this pilot study, so further studies of larger sample sizes are needed to clarify the relationship between the G1195A polymorphism and clinicopathological characteristics in prostate cancer. The polymorphism also merits further study as a potential genomic marker for the early detection of prostate cancer. Also, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly known as statins, have been hypothesized to be anti-inflammatory in some studies and function synergistically with drugs containing COX2 activity such as aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) [25, 26]. Therefore, statins may prevent the prostate cancer susceptibility in the group of A variant in particular. More sophisticated gene–gene and gene–environment interactions [27], such as the effects of environmental exposure to specific carcinogens, together with genotype–phenotype correlations should also be investigated in future studies.
References
Committee for Establishment of the Guidelines on Screening for Prostate Cancer. Japanese Urological Association: updated Japanese Urological Association guidelines on prostate-specific antigen-based screening for prostate cancer in 2010. Int J Urol. 2010;17(10):830–8.
Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–26.
McArdle PA, Mir K, Almushatat AS, Wallace AM, Underwood MA, McMillan DC. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol Int. 2006;77:127–9.
Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–18.
Serefoglou Z, Yapijakis C, Nkenke E, Vairaktaris E. Genetic association of cytokine DNA polymorphisms with head and neck cancer. Oral Oncol. 2008;44:1093–9.
Howell WM, Rose-Zerilli MJ. Cytokine gene polymorphisms, cancer susceptibility, and prognosis. J Nutr. 2007;137:194–9.
Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1995;1299:125–40.
Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–66.
Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73.
Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS. COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer. 2007;97:557–61.
Panguluri RC, Long LO, Chen W, Wang S, Coulibaly A, Ukoli F, et al. COX-2 gene promoter haplotypes and prostate cancer risk. Carcinogenesis. 2004;25:961–6.
Shahedi K, Lindstrom S, Zheng SL, Wiklund F, Adolfsson J, Sun J, et al. Genetic variation in the COX-2 gene and the association with prostate cancer risk. Int J Cancer. 2006;119:668–72.
Fernandez P, de Beer PM, van der Merwe L, Heyns CF. COX-2 promoter polymorphisms and the association with prostate cancer risk in South African men. Carcinogenesis. 2008;29:2347–50.
Edwards SM, Eeles RA. Unravelling the genetics of prostate cancer. Am J Med Genet C. 2004;129:65–73.
Bau DT, Tsai MH, Huang CY, Lee CC, Tseng HC, Lo YL, et al. Relationship between polymorphisms of nucleotide excision repair genes and oral cancer risk in Taiwan: evidence for modification of smoking habit. Chin J Physiol. 2007;50:294–300.
Chang CH, Wang RF, Tsai RY, Wu HC, Wang CH, Tsai CW, et al. Significant association of XPD codon 312 single nucleotide polymorphism with bladder cancer susceptibility in Taiwan. Anticancer Res. 2009;29:3903–7.
Wu GY, Hasenberg T, Magdeburg R, Bönninghoff R, Sturm JW, Keese M. Association between EGF, TGF-beta1, VEGF gene polymorphism and colorectal cancer. World J Surg. 2009;33:124–9.
Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–8.
Zhang X, Miao X, Tan W, Ning B, Liu Z, Hong Y, et al. Identification of functional genetic variants in cyclooxygenase-2 and their association with risk of esophageal cancer. Gastroenterology. 2005;129:565–76.
Gao J, Ke Q, Ma HX, Wang Y, Zhou Y, Hu ZB, et al. Functional polymorphisms in the cyclooxygenase 2 (COX-2) gene and risk of breast cancer in a Chinese population. J Toxicol Environ Health A. 2007;70:908–15.
Chiang SL, Chen PH, Lee CH, Ko AM, Lee KW, Lin YC, et al. Up-regulation of inflammatory signallings by areca nut extract and role of cyclooxygenase-2 -1195G>A polymorphism reveal risk of oral cancer. Cancer Res. 2008;68:8489–98.
Peters WH, Lacko M, Te Morche RH, Voogd AC, Oude Ophius MB, Manni JJ. COX-2 polymorphisms and the risk for head and neck cancer in white patients. Head Neck. 2009;31:938–43.
Wu HC, Chang CH, Ke HL, Chang WS, Cheng HN, Lin HH, et al. Association of cyclooxygenase 2 polymorphic genotypes with prostate cancer in Taiwan. Anticancer Res. 2011;31(1):221–5.
Mandal RK, Mittal RD. Polymorphisms in COX-2 gene influence prostate cancer susceptibility in a northern Indian cohort. Arch Med Res. 2011;42(7):620–6.
Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case–control study. Int J Cancer. 2007;121:1325–30.
Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7.
Wark PA, Van der Kuil W, Ploemacher J, Van Muijen GN, Mulder CJ, Weijenberg MP, et al. Diet, lifestyle and risk of K-ras mutation-positive and -negative colorectal adenomas. Int J Cancer. 2006;119:398–405.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugie, S., Tsukino, H., Mukai, S. et al. Cyclooxygenase 2 genotypes influence prostate cancer susceptibility in Japanese Men. Tumor Biol. 35, 2717–2721 (2014). https://doi.org/10.1007/s13277-013-1358-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-013-1358-y