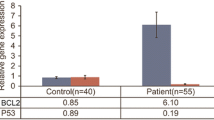
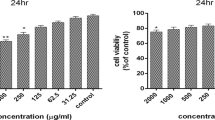
Abstract
The BCL2 family of proteins includes apoptosis-related molecules involved in normal physiology, as well as cancer pathology. Members of our team have discovered and cloned the novel gene BCL2L12, which codes for a protein member of the BCL2 family. The BCL2L12 expression has been studied extensively in various types of cancer and its important clinical value has been underlined. The main objective of this study is the relative quantification of the mRNA expression of the apoptosis-related genes BCL2, BAX and BCL2L12 in gastric cancer cells, following treatment with anticancer drugs. Gastric adenocarcinoma cells AGS were treated with various concentrations of the chemical substances cisplatin, etoposide and taxol for three time periods. Cell viability was examined by using the MTT assay. Total RNA was extracted and reverse transcribed into cDNA. A highly sensitive, quantitative real-time PCR method was developed based on the SYBR Green chemistry, for the proper mRNA quantification. GAPDH was used as a housekeeping gene. Relative quantification analysis was performed by using the comparative CT method (\( 2^{−{\text{DDC}}_{\text{T}}} \)). Treatment of AGS cells with 10 μM cisplatin, 0.5 μM etoposide and 10 nM taxol affected the BCL2, BAX and BCL2L12 mRNA levels, compared to the untreated cells. Cisplatin and etoposide induced a major down-regulation in the BCL2 mRNA levels after 72 h of treatment, while the BAX mRNA levels were slightly up-regulated. Moreover, taxol had an up-regulating effect on both BCL2 and BAX transcript levels after 48 h of incubation. Chemotherapy had a much smaller effect on the BCL2L12 expression levels, eventually characterised by a small down-regulation.



Similar content being viewed by others
Abbreviations
- BCL2:
-
B-cell CLL/lymphoma 2
- BCL2L12 :
-
BCL2 like gene 12
- BH domain:
-
BCL2 homology domains
- CA 19-9:
-
Carbohydrate antigen 19-9
- CA 72-4:
-
Cancer antigen 72-4
- CEA:
-
Carcinoembryonic antigen
- CLL:
-
Chronic lymphocytic leukaemia
- DFS:
-
Disease-free survival
- H. pylori :
-
Helicobacter pylori
- IRF3 :
-
Interferon regulatory factor 3 gene
- PRMT1/HRMT1L2 :
-
Protein arginine methyltransferase 1 gene
- SSCP:
-
Single-strand conformation polymorphism
References
Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57.
Thomadaki H, Scorilas A. Bcl2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1–67.
Petros AM, Olejniczak ET, Fesik SW. Structural biology of the bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94.
Adams JM, Cory S. The bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6.
Hanada M, Aime-Sempe C, Sato T, Reed JC. Structure–function analysis of bcl-2 protein. Identification of conserved domains important for homodimerization with bcl-2 and heterodimerization with bax. J Biol Chem. 1995;270:11962–9.
Zivny J, Klener Jr P. Pytlik R, Andera L: The role of apoptosis in cancer development and treatment: focusing on the development and treatment of hematologic malignancies. Curr Pharm Des. 2010;16:11–33.
Kirkin V, Joos S, Zornig M. The role of bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–49.
Apte SS, Mattei MG, Olsen BR. Mapping of the human bax gene to chromosome 19q13.3-q13.4 and isolation of a novel alternatively spliced transcript, bax delta. Genomics. 1995;26:592–4.
Oltvai ZN, Korsmeyer SJ. Checkpoints of dueling dimers foil death wishes. Cell. 1994;79:189–92.
Scorilas A, Kyriakopoulou L, Yousef GM, Ashworth LK, Kwamie A, Diamandis EP. Molecular cloning, physical mapping, and expression analysis of a novel gene, bcl2l12, encoding a proline-rich protein with a highly conserved bh2 domain of the bcl-2 family. Genomics. 2001;72:217–21.
Fenogilo-Preiser C, Carneiro F, Correa P. Gastric carcinoma. In: Hamilton S, Aaltonin L, editors. Pathology and genetics tumors of the digestive system, vol 1. Lyon: Lyon Press; 2000.
Partridge M, Gaballah K, Huang X. Molecular markers for diagnosis and prognosis. Cancer Metastasis Rev. 2005;24:71–85.
Schwartz GK. Invasion and metastases in gastric cancer: in vitro and in vivo models with clinical correlations. Semin Oncol. 1996;23:316–24.
Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71:127–64.
Ebert MP, Rocken C. Molecular screening of gastric cancer by proteome analysis. Eur J Gastroenterol Hepatol. 2006;18:847–53.
Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–3.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta c(t)) method. Methods. 2001;25:402–8.
Katsumata M, Siegel RM, Louie DC, Miyashita T, Tsujimoto Y, Nowell PC, et al. Differential effects of Bcl-2 on T and B cells in transgenic mice. Proc Natl Acad Sci USA. 1992;89:11376–80.
McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger U, McKearn JP, et al. Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88.
Bairey O, Zimra Y, Shaklai M, Okon E, Rabizadeh E. Bcl-2, Bcl-x, Bax, and Bak expression in short- and long-lived patients with diffuse large B-cell lymphomas. Clin Cancer Res. 1999;5:2860–6.
Binder C, Marx D, Overhoff R, Binder L, Schauer A, Hiddemann W. Bcl-2 protein expression in breast cancer in relation to established prognostic factors and other clinicopathological variables. Ann Oncol. 1995;6:1005–10.
Campana D, Coustan-Smith E, Manabe A, Buschle M, Raimondi SC, Behm FG, et al. Prolonged survival of B-lineage acute lymphoblastic leukemia cells is accompanied by overexpression of bcl-2 protein. Blood. 1993;81:1025–31.
Campos L, Sabido O, Sebban C, Charrin C, Bertheas MF, Fiere D, et al. Expression of bcl-2 proto-oncogene in adult acute lymphoblastic leukemia. Leukemia. 1996;10:434–8.
Flohil CC, Janssen PA, Bosman FT. Expression of bcl-2 protein in hyperplastic polyps, adenomas, and carcinomas of the colon. J Pathol. 1996;178:393–7.
Fontanini G, Vignati S, Bigini D, Mussi A, Lucchi M, Angeletti CA, et al. Bcl-2 protein: a prognostic factor inversely correlated to p53 in non-small-cell lung cancer. Br J Cancer. 1995;71:1003–7.
Karakas T, Maurer U, Weidmann E, Miething CC, Hoelzer D, Bergmann L. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann Oncol. 1998;9:159–65.
Laudanski J, Chyczewski L, Niklinska WE, Kretowska M, Furman M, Sawicki B, et al. Expression of bcl-2 protein in non-small cell lung cancer: correlation with clinicopathology and patient survival. Neoplasma. 1999;46:25–30.
Ramsay JA, From L, Kahn HJ. Bcl-2 protein expression in melanocytic neoplasms of the skin. Mod Pathol. 1995;8:150–4.
Schorr K, Li M, Krajewski S, Reed JC, Furth PA. Bcl-2 gene family and related proteins in mammary gland involution and breast cancer. J Mammary Gland Biol Neoplasia. 1999;4:153–64.
Tsuji M, Murakami Y, Kanayama H, Sano T, Kagawa S. Immunohistochemical analysis of Ki-67 antigen and Bcl-2 protein expression in prostate cancer: effect of neoadjuvant hormonal therapy. Br J Urol. 1998;81:116–21.
Konturek PC, Konturek SJ, Sulekova Z, Meixner H, Bielanski W, Starzynska T, et al. Expression of hepatocyte growth factor, transforming growth factor alpha, apoptosis related proteins Bax and Bcl-2, and gastrin in human gastric cancer. Aliment Pharmacol Ther. 2001;15:989–99.
Krajewska M, Fenoglio-Preiser CM, Krajewski S, Song K, Macdonald JS, Stemmerman G, et al. Immunohistochemical analysis of Bcl-2 family proteins in adenocarcinomas of the stomach. Am J Pathol. 1996;149:1449–57.
Lauwers GY, Scott GV, Karpeh MS. Immunohistochemical evaluation of bcl-2 protein expression in gastric adenocarcinomas. Cancer. 1995;75:2209–13.
Panani AD. Cytogenetic and molecular aspects of gastric cancer: clinical implications. Cancer Lett. 2008;266:99–115.
Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003;97:545–53.
Richardson A, Kaye SB. Pharmacological inhibition of the bcl-2 family of apoptosis regulators as cancer therapy. Curr Mol Pharmacol. 2008;1:244–54.
Thomadaki H, Scorilas A. Molecular profile of the bcl2 family of the apoptosis related genes in breast cancer cells after treatment with cytotoxic/cytostatic drugs. Connect Tissue Res. 2008;49:261–4.
Floros KV, Talieri M, Scorilas A. Topotecan and methotrexate alter expression of the apoptosis-related genes BCL2, FAS and BCL2L12 in leukemic HL-60 cells. Biol Chem. 2006;387:1629–33.
Floros KV, Thomadaki H, Katsaros N, Talieri M, Scorilas A. mRNA expression analysis of a variety of apoptosis-related genes, including the novel gene of the BCL2-family, BCL2L12, in HL-60 leukemia cells after treatment with carboplatin and doxorubicin. Biol Chem. 2004;385:1099–103.
Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604.
Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4.
Kaneuchi M, Yamashita T, Shindoh M, Segawa K, Takahashi S, Furuta I, et al. Induction of apoptosis by the p53-273l (Arg → Leu) mutant in HSC3 cells without transactivation of p21Waf1/Cip1/Sdi1 and bax. Mol Carcinog. 1999;26:44–52.
Salah-eldin A, Inoue S, Tsuda M, Matsuura A. Abnormal intracellular localization of Bax with a normal membrane anchor domain in human lung cancer cell lines. Jpn J Cancer Res. 2000;91:1269–77.
Sturm I, Papadopoulos S, Hillebrand T, Benter T, Luck HJ, Wolff G, et al. Impaired BAX protein expression in breast cancer: mutational analysis of the BAX and the p53 gene. Int J Cancer. 2000;87:517–21.
Gil J, Yamamoto H, Zapata JM, Reed JC, Perucho M. Impairment of the proapoptotic activity of Bax by missense mutations found in gastrointestinal cancers. Cancer Res. 1999;59:2034–7.
Komatsu K, Suzuki S, Shimosegawa T, Miyazaki JI, Toyota T. Cre-loxP-mediated bax gene activation reduces growth rate and increases sensitivity to chemotherapeutic agents in human gastric cancer cells. Cancer Gene Ther. 2000;7:885–92.
Floros KV, Thomadaki H, Florou D, Talieri M, Scorilas A. Alterations in mRNA expression of apoptosis-related genes BCL2, BAX, FAS, caspase-3, and the novel member BCL2L12 after treatment of human leukemic cell line HL60 with the antineoplastic agent etoposide. Ann N Y Acad Sci. 2006;1090:89–97.
Stegh AH, Chin L, Louis DN, DePinho RA. What drives intense apoptosis resistance and propensity for necrosis in glioblastoma? A role for Bcl2l12 as a multifunctional cell death regulator. Cell Cycle. 2008;7:2833–9.
Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2l12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA. 2008;105:10703–8.
Stegh AH, Kim H, Bachoo RM, Forloney KL, Zhang J, Schulze H, et al. Bcl2l12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev. 2007;21:98–111.
Stegh AH, Brennan C, Mahoney JA, Forloney KL, Jenq HT, Luciano JP, et al. Glioma oncoprotein bcl2l12 inhibits the p53 tumor suppressor. Genes Dev. 2010;24:2194–204.
Nakajima A, Nishimura K, Nakaima Y, Oh T, Noguchi S, Taniguchi T, et al. Cell type-dependent proapoptotic role of bcl2l12 revealed by a mutation concomitant with the disruption of the juxtaposed irf3 gene. Proc Natl Acad Sci USA. 2009;106:12448–52.
Hong Y, Yang J, Wu W, Wang W, Kong X, Wang Y, et al. Knockdown of BCL2L12 leads to cisplatin resistance in MDA-MB-231 breast cancer cells. Biochim Biophys Acta. 2008;1782:649–57.
Hong Y, Yang J, Chi Y, Wang W, Wu W, Yun X, et al. BCL2L12A localizes to the cell nucleus and induces growth inhibition through G2/M arrest in CHO cells. Mol Cell Biochem. 2010;333:323–30.
Talieri M, Diamandis EP, Katsaros N, Gourgiotis D, Scorilas A. Expression of BCL2L12, a new member of apoptosis-related genes, in breast tumors. Thromb Haemost. 2003;89:1081–8.
Thomadaki H, Talieri M, Scorilas A. Prognostic value of the apoptosis related genes BCL2 and BCL2L12 in breast cancer. Cancer Lett. 2007;247:48–55.
Mathioudaki K, Scorilas A, Papadokostopoulou A, Xynopoulos D, Arnogianaki N, Agnanti N, et al. Expression analysis of BCL2L12, a new member of apoptosis-related genes, in colon cancer. Biol Chem. 2004;385:779–83.
Kontos CK, Papadopoulos IN, Scorilas A. Quantitative expression analysis and prognostic significance of the novel apoptosis-related gene BCL2L12 in colon cancer. Biol Chem. 2008;389:1467–75.
Papageorgiou SG, Kontos CK, Pappa V, Thomadaki H, Kontsioti F, Dervenoulas J, et al. The novel member of the BCL2 gene family, BCL2L12, is substantially elevated in chronic lymphocytic leukemia patients, supporting its value as a significant biomarker. Oncologist. 2011;16:1280–91.
Fendri A, Kontos CK, Khabir A, Mokdad-Gargouri R, Scorilas A. BCL2L12 is a novel biomarker for the prediction of short-term relapse in nasopharyngeal carcinoma. Mol Med. 2011;17:163–71.
Florou D, Papadopoulos IN, Scorilas A. Molecular analysis and prognostic impact of the novel apoptotic gene BCL2L12 in gastric cancer. Biochem Biophys Res Commun. 2010;391:214–8.
Floros KV, Thomadaki H, Lallas G, Katsaros N, Talieri M, Scorilas A. Cisplatin-induced apoptosis in HL-60 human promyelocytic leukemia cells: differential expression of BCL2 and novel apoptosis-related gene BCL2L12. Ann N Y Acad Sci. 2003;1010:153–8.
Thomadaki H, Floros KV, Scorilas A. Molecular response of HL-60 cells to mitotic inhibitors vincristine and taxol visualized with apoptosis-related gene expressions, including the new member BCL2L12. Ann N Y Acad Sci. 2009;1171:276–83.
Thomadaki H, Scorilas A. Breast cancer cells response to the antineoplastic agents cisplatin, carboplatin, and doxorubicin at the mRNA expression levels of distinct apoptosis-related genes, including the new member, BCL2L12. Ann N Y Acad Sci. 2007;1095:35–44.
Thomadaki H, Talieri M, Scorilas A. Treatment of MCF-7 cells with taxol and etoposide induces distinct alterations in the expression of apoptosis-related genes BCL2, BCL2L12, BAX, CASPASE-9 and FAS. Biol Chem. 2006;387:1081–6.
Thomadaki H, Scorilas A. Molecular profile of breast versus ovarian cancer cells in response to treatment with the anticancer drugs cisplatin, carboplatin, doxorubicin, etoposide and taxol. Biol Chem. 2008;389:1427–34.
Zhuo Z, Zhang L, Mu Q, Lou Y, Gong Z, Shi Y, et al. The effect of combination treatment with docosahexaenoic acid and 5-fluorouracil on the mRNA expression of apoptosis-related genes, including the novel gene BCL2L12, in gastric cancer cells. In Vitro Cell Dev Biol Anim. 2009;45:69–74.
Korbakis D, Scorilas A. Treatment of gastric cancer cells with 5-fluorouracil/leucovorin and irinotecan induces distinct alterations in the mRNA expression of the apoptosis-related genes, including the novel gene BCL2L12. Tumour Biol. 2009;30:100–7.
Acknowledgements
The project was supported by a PENED grant co-funded by the European Union–European Social Funds (75% from the European Union), National Resources (25% from governmental funds)–Ministry of Development–General Secretariat for Research & Technology of Greece and ELPEN A.E. through EPAN.M.8.3—3rd Community Support Programme.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Korbakis, D., Scorilas, A. Quantitative expression analysis of the apoptosis-related genes BCL2, BAX and BCL2L12 in gastric adenocarcinoma cells following treatment with the anticancer drugs cisplatin, etoposide and taxol. Tumor Biol. 33, 865–875 (2012). https://doi.org/10.1007/s13277-011-0313-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-011-0313-z