Abstract
Purpose
To evaluate the relationship between FDG uptake and prognostic factors of breast cancer such as hormone receptors (estrogen and progesterone), expression of c-erbB-2, axillary lymph node status, tumor histology, grade and size.
Materials and methods
Between May 2009 and February 2011; 79 patients (mean age ± SD: 52.9 ± 13.9 years) with biopsy proven breast cancer underwent F-18 FDG PET/CT scanning for staging. Patients with excisional biopsy or neoadjuvant chemotherapy were excluded from the study. Histological types included were invasive ductal carcinoma (n = 68), invasive lobular carcinoma (n = 2), and invasive ductal plus lobular mixed carcinoma (n = 9). Maximum standardized uptake values (SUVmax) were compared with estrogen (ER) and progesterone receptors (PR), expression of c-erbB-2, as well as tumor grade and tumor size. For the evaluation of relationship between tumor SUVmax values and prognosticators such as hormone receptors, tumor histologic grade, and tumor size, statistical analyses were performed using Student t test, Mann–Whitney U Test and Pearson correlation coefficient and p values of less than 0.05 were considered to indicate statistically significant differences.
Results
All primary breast neoplasms were detected by PET/CT scanner. The mean SUVmax values and breast cancer tumor sizes ranged from 2.09 to 39.0 and 0.7 to 10 cm, respectively. Tumors with negative ER [(n = 19); SUVmax median (min–max): 15 (2.09–39.0)] were associated with higher SUVmax values (p = 0.01). Tumors with overexpression of C-erbB-2 [(n = 28); SUVmax median (min–max): 16.0 (5.0-39.0)]; tumor grade 3 [(n = 25); SUVmax median (min–max): 15 (6.43–39)]; axillary lymph node involvement [(n = 60); SUVmax median (min–max): 13.61 (4.0–39.0)]; tumor histopathology and increased tumor size were associated with higher maximum standardized uptake values. However, PR did not show any relationship with SUVmax values.
Conclusion
In the present report, strong relationships were detected between the negativity of ER, overexpression of c-erbB-2, tumor grade, tumor size, histopathology, axillary lymph node involvement and SUVmax values. Accordingly, we believe that SUVmax values obtained with 18F-FDG PET/CT may provide some information about tumor biology of breast cancer.
Similar content being viewed by others
Introduction
Breast cancer is the most frequent malignancy and the second leading cause of cancer death in women in western countries [1]. In patients with breast cancer, preoperative staging of locally advanced tumors is extremely important, as it may affect treatment decision. For this purpose, the use of 18F-FDG PET/CT in preoperative management of early-stage breast cancer was suggested since it has been established as a useful technique for clinical staging in some human cancers such as lung carcinoma, pancreatic adenocarcinoma and lymphoma [2].
An advantage of PET over other conventional oncologic imaging is the ability to quantify functional tumor biology. The most commonly used radiotracer in PET, 18F-FDG, targets tumor cells that exhibit increased glucose metabolism [3]. In breast cancer, 18F-FDG PET/CT study has been used for tumor detection and staging to obtain long-term prognostic information, and to identify and monitor the response of the primary tumor to neoadjuvant chemotherapy [4–6].
On the other hand, hormone receptors (HR) and c-erbB-2 status provide prognostic information about the tumor, but more importantly, represent predictive markers that directly impact therapeutic decision making: HR predict a response to hormonal therapy; c-erbB-2 positivity is predictive of a response to the humanized anti-c-erbB-2 monoclonal antibody, trastuzumab, which significantly improves disease-free and overall survival compared with chemotherapy alone in patients with c-erbB-2 positive metastatic breast cancer and in patients in the adjuvant setting. Furthermore, c-erbB-2 status may predict sensitivity to certain cytotoxic drugs and antiestrogens [7–10]. Several studies have investigated the correlation between the intensity of 18F-FDG uptake and some of the histological and biological characteristics such as tumor type, grade, hormonal-receptor status and c-erbB-2 status [11–17]. Accordingly, the aim of the present retrospective study using the prospectively collected data was to determine the possible relationship between FDG uptake and clinical and biological prognostic parameters in breast cancer.
Methods
Patient population
Between May 2009 and February 2011, 79 patients (mean age ± SD: 52.9 ± 13.9 years) with true-cut biopsy proven breast cancer underwent 18F-FDG PET/CT scanning for staging. Tumor size and T stage were assessed by clinical examination, ultrasonography, mammogram and magnetic resonance imaging (MRI). Patients who had excisional biopsy were excluded from the study because of higher incidence of inflammatory complications that may interfere with tumor imaging with 18F-FDG PET/CT. None of the patients had received chemo- or radiation therapy before undergoing 18F-FDG PET/CT for preoperative staging. Patients with uncontrolled diabetes mellitus (DM) were also not included to the study. Maximum standardized uptake values (SUVmax) were compared with estrogen (ER) and progesterone (PR) receptors, expression of c-erbB-2, axillary involvement, as well as tumor grade and tumor size.
Imaging protocol
All patients were given oral contrast 12 h before the start of the PET/CT study. All patients were fasted for at least 6 h prior to imaging, and blood glucose levels were <170 mg/dl at the time of the tracer injection. Patients received an intravenous injection of 370–550 MBq of 18F-FDG and then rested for approximately 60 min before undergoing imaging. To avoid FDG uptake in muscles during the distribution phase of injected FDG, all patients were instructed to avoid talking, chewing or any muscular activity. After the patients were positioned supine on the scanner, an initial scout image was acquired for defining the examination range for the PET/CT image acquisition. First, low-dose CT was performed (140 kV and app. 50 mAs) without any specific breath-holding instructions. Scanning from the top of the skull through the upper thighs was performed in a single step with the patient in a supine position. CT data were used for attenuation correction. Immediately following CT, a PET emission scan was acquired in the same position. Six to eight bed positions were used, with an acquisition time of 3 min for each bed position.
Immunochemistry
Sections 3–5 μm thick were obtained from the paraffin blocks and reacted in an automatic Ventana immunostainer (Ventana XT, Tucson, AZ, USA) with the following monoclonal antibodies raised against: ER (NeoMarkers, clone SP1, dil.1:200, Fremont, CA, USA); PR (Novocastra, clone 16, dil.1:200, Newcastle upon Tyne, UK); c-erbB-2 (Thermo scientific, clone SP3, dil. 1:100, Vantaa, Finland); Ki-67 (NeoMarkers, clone SP6, dil.1:200, Fremont, CA, USA). Receptor status was scored as positive or negative using a cutoff of 10% nuclear immunostaining for estrogen and progesterone and 10% nuclear or membrane staining for c-erbB-2. C-erbB-2 was categorized as scores 0 or 1+ versus score 2+ or 3+, and nodal status was categorized as nodal metastasis positive versus negative. Histologic grade was determined using the modified Scarff–Bloom–Richardson system, as for invasive carcinoma [13].
Statistics
For the evaluation of relationship between tumor SUVmax values and prognosticators such as hormone receptors, c-erbB-2, menopausal status, and axilla involvement, statistical analyses were performed using Student t test and p values of <0.05 were considered to indicate statistically significant differences. The Mann–Whitney U test was used to determine differences of SUVmax among triple negativity, receptors negative and cebB-2 positivity, between invasive ductal and ductal plus lobular mixed carcinomas, and between grade 2 and grade 3 carcinomas. The correlation between tumor size and SUVmax values were determined using Pearson correlation coefficient.
Results
Seventy-nine patients with invasive breast carcinoma before neoadjuvant chemotherapy were included in the present study. The median age was 50 years (range 21–81 years); whereas the median tumor size was 3 cm (range 0.7–10 cm). Thirty-four patients (43.0%) were premenopausal and 45 patients (56.9%) were postmenopausal at the time of diagnosis. The present study revealed no significant difference between menopausal status and SUVmax values.
Histological types included were invasive ductal carcinoma (n = 68), invasive lobular carcinoma (n = 2), and invasive ductal plus lobular (mixed) carcinoma (n = 9). Because of the limited number, patients with pure invasive lobular carcinoma were excluded from the statistical analysis in terms of comparison of histological subtypes with SUVmax values. The mean SUVmax values of patients with invasive ductal carcinoma [SUVmax median (min–max): 12 (2–39)] were significantly different from patients with mixed (ductal plus lobular) carcinoma [SUVmax median (min–max): 6.20 (4–17); p < 0.001, Mann–Whitney U test].
Among 79 patients, the tumor grade value was available in 49 patients. One patient with grade 1 tumor was left out of analysis in this cohort. Patients with grade 3 breast carcinomas (n = 25) had SUVmax median (min–max): 15 (6.43–39) versus 7.0 (2.0–20.0) in grade 2 tumors (p < 0.001, Mann–Whitney U test).
The mean SUVmax values and breast cancer tumor sizes ranged from 2.09 to 39.0 and 0.7 to 10.0 cm, respectively. Patients with increased tumor sizes were associated with higher maximum standard uptake values (Fig. 1). Positive correlation (r = 0.252; p < 0.025; Pearson correlation coefficient) was observed between FDG uptake and tumor size.
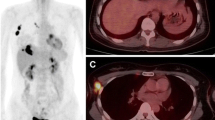
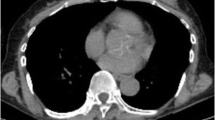
A 77-year-old woman underwent F-18 FDG PET/CT imaging for breast cancer staging. Her biopsy results were intraductal carcinoma, estrogen and progesterone receptors were negative and c-erbB-2 positive (+++). She had 47 × 77 mm mass in upper quadrant of left breast in CT. In addition she had hypermetabolic left axillary, supraclavicular and left internal mammarian lymph nodes. Primary tumor SUVmax value was determined as 36.9
All primary breast neoplasms were detected by PET/CT scanner. Among these, ER was positive in 56 of 79 (70.8%) patients and PR was positive in 43 of 79 (54.4%) patients. Over expression of c-erbB-2 such as 2+/3+ was found in 28 of 79 (35.4%) patients. Nine patients (11.3%) had a triple-negative tumor. Student’s t test revealed no significant differences between the level of SUVmax and PR negativity [SUVmax median (min–max): 11.84 (2.09–39)] and Mann–Whitney U test revealed no significant differences between the level of SUVmax and triple negativity [SUVmax median (min–max): 11.59 (2.0–39)]. However, the level of SUVmax and ER negativity and overexpression of c-erbB-2 were significantly different [SUVmax median (min–max): 15.0 (2.09–39.0); 16.0 (5.0-39.0), respectively]. The clinicopathological features of the 79 patients are outlined in Table 1.
Axillary lymph node involvement was demonstrated in 60 of 79 (75.9%) patients with PET/CT study. The level of SUVmax was higher in the tumor group with metastatic lymph nodes than in the group without metastatic lymph nodes [SUVmax median (min–max): 13.6 (4–39) vs. 6.36 (2–20); p < 0.001, Mann–Whitney U test].
After a mean follow-up of 18.1 ± 5.2 months, 11 (13.9%) patients were lost to follow-up; whereas 68 (86.0%) patients were seen on regular basis. Of 68 patients, 6 (8.8%) were died due to breast cancer, 5 (7.3%) experienced progression of disease and in 57 (83.8%) patients no progression was noted. The mean SUVmax values of patients who died due to breast cancer were not different from the patients without disease progression [SUVmax median (min–max): 12.0 (7–23) vs. 12.0 (2–39); p = 0.841, Mann–Whitney U test]. Similarly, no significant difference in terms of SUVmax values was detected between patients with and without disease progression [SUVmax median (min–max): 11.0 (6.2–27) vs. 12.0 (2–39); p = 0.79, Mann–Whitney U test].
Discussion
PET-CT has recently been introduced in tumor staging of breast cancer in conjunction with staging methods such as bone scintigraphy and ultrasonography or CT scan for breast, axilla and liver. There is evidence in the literature that, PET-CT scan may significantly contribute to the staging of the breast cancer, in addition to one or more of the above-mentioned conventional methods [18, 19]. In theory, 18F-FDG uptake in breast cancer usually visualizes metabolic pathways and hence can estimate its behavior and prognosis [11, 15]. However, it is also known that breast carcinomas display a considerable variation in FDG uptake and characterization of small lesions (less than 0.8 cm) is less reliable probably due to partial volume effect and low metabolic activity which may also indicate inflammatory lesions [14]. Despite these limitations of PET-CT, some studies found a positive relationship between SUVmax values and tumor sizes [2]. The present study also confirmed that increased tumor sizes were associated and correlated with higher maximum standard uptake values. However, it is worth mentioning that others found no significant relationship between tumor size and SUVmax values, especially in lesions ≤2 cm [15]. This discrepancy in the existing literature may be explained by the histological (necrotic or fibrotic tissue) and immunohistochemical differences (ER and PR expression) between the tumors that may influence the SUVmax values by effecting on tumoral microenvironment.
In breast cancer, tumor histopathology indicates that lobular component is associated with lower FDG uptake than ductal breast carcinomas [2, 13, 15–17]. Although in our series only 2 patients had invasive lobular carcinoma, we noted that mixed (lobular plus ductal) carcinoma group had significantly lower SUVmax values in comparison with pure ductal carcinoma. Similarly, Buck et al. [16] reported in a prospective study that there is significantly lower FDG uptake in lobular than in ductal breast cancer which is thought to be due to increased proliferative index in ductal carcinoma assessed with Ki-67 labeling index. Meanwhile, Avril et al. found that histopathology (ductal vs. lobular) and tumor growth pattern (nodular vs. diffuse) were correlated with SUVmax levels; whereas a weak and no relationship was detected between SUVmax values and percentage of tumor cells and size of the tumors, respectively. Meanwhile, the authors addressed the importance of finding lower SUVmax values since these lesions may be the sign of lobular carcinoma which is known to have higher false-negative rates [11].
Several reports including ours found a significant relationship between FDG uptake and pathologic grade which is due to the finding that less differentiated tumors with higher tumor growth rates exhibits higher FDG uptake [11]. This may be due to high levels of Glut-1 at the membrane and increased level of hexokinase in their cytoplasm, encountered in high grade human cancers including breast cancer [4, 5, 15, 20, 21]. Consequently, in the light of the current literature, we think that mass in the breast with high SUVmax values may indicate a high grade, high stage tumor in whom identification of nodal or distant metastasis have utmost importance since these patients will receive upfront chemotherapy. This consideration is in line with the study by Duch et al. who reported that a cut-off ΔSUVmax value of 40% before and after neoadjuvant chemotherapy can predict response to therapy with a sensitivity of 77% and a specificity of 80% [6].
The HER-2/neu oncogene (c-erbB-2) is a member of the erbB-like oncogene family, and is related to, but distinct from, the epidermal growth factor receptor. Amplification of the c-erbB-2 receptor is a significant predictor of both overall survival and time to relapse in patients with breast cancer. Moreover, c-erbB-2 amplification has greater prognostic value than most currently used prognostic factors, including hormonal-receptor status in lymph node-positive disease [22]. These data indicate that this gene may play a role in the biologic behavior and/or pathogenesis of human breast cancer. Determination of ER and PR and also c-erbB-2 receptor in tumor cells is essential for appropriate hormone therapy and the use of targeted adjuvant therapy. In the present study, we found a significant correlation between SUVmax values and the existence of ER and c-erbB-2 receptors. On the other hand, PR and triple negativity did not show a positive correlation with SUVmax values. Osborne et al. [2] demonstrated that a relationship existed between lesion 18F-FDG uptake and ER status in preoperative advanced locoregional breast cancer. Furthermore, PR and c-erbB-2 status did not correlate with 18F-FDG uptake. Similar outcomes were also obtained by Avril et al. and Buck et al. [11, 16]. On the contrary, Basu et al. [23] reported that triple-negative breast tumors were associated with a higher FDG uptake in comparison with ER+/PR+/c-erbB-2− tumors. Meanwhile, the authors noted that 18F-FDG-PET had 100% sensitivity for determining triple-negative breast tumors and was more prominent if tumor diameter was >2 cm. Consequently, the relationship between the hormonal status and c-erbB-2 receptor positivity and FDG uptake parameters is not clear in the literature probably due to low power of the studies.
Axillary lymph node status is one of the most significant parameters of breast cancer. Ueda et al. reported in a prospective study including 152 patients with breast surgery including sentinel node biopsy or axillary lymph node dissection that the mean level of SUVmax values was higher in the tumor group with metastatic lymph nodes than in the group without metastatic lymph nodes [15]. Similar outcome was also found by Mavi et al. [14] in a prospective study including presurgical 240 consecutive patients with biopsy proven breast cancer. The present study also confirmed the outcomes of the former studies that tumors with axillary lymph node metastasis had higher SUVmax values in comparison with tumors without axillary lymph node metastasis. However, limited number of patients in our study prevents giving a cut-off SUVmax value for estimating lymph node status in breast cancer patients. However, Straver et al. [24] reported that the sensitivity and specificity of FDG PET/CT in detecting axillary involvement were 97 and 100%, respectively, with SUVmax value ≥2.5 or a tumor to background ratio ≥5. The sensitivity rate reported by others varies between 20 and 94% depending on the other biological (triple-negative tumors) and clinical prognosticators (>grade 3 tumors) [25, 26].
The present study has some limitations that merit mentioning. Firstly, the limited number of patients with invasive lobular carcinoma disables comparison of this tumor with invasive ductal carcinoma in terms of SUVmax values. Secondly, complete surgical removal of the tumor for estimating the total tumor volume is lacking. Thus, tumor diameter calculated with conventional imaging modalities was used as an estimate of tumor volume for investigating the relationship between tumor load and SUVmax values. In addition, the relationship between the FDG uptake parameters and treatment outcomes and survival is beyond the scope of this study to perform survival analysis. However, a possible relationship such as patients with SUVmax value 4.0 or less (or tumor diameter <2 cm) has tendency of lower 10-year relapse rate and mortality in comparison with patients with SUVmax values more than 4.0 (or tumor diameter ≥2 cm) addressed by Ueda et al. [15] should definitely be identified with further studies.
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30.
Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010;51:543–50.
Dunnwald LK, Doot RK, Specht JM, Gralow JR, Ellis GK, Livingston RB, et al. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17:2400–9.
Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer. 1998;82:2227–34.
Berriolo-Riedinger A, Touzery C, Riedinger JM, Toubeau M, Coudert B, Arnould L, et al. 18F-FDG-PET predicts complete pathological response of breast cancer to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2007;34:1915–24.
Duch J, Fuster D, Muñoz M, Fernández PL, Paredes P, Fontanillas M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–7.
Borg A, Tandon AK, Sigurdsson H, Clark GM, Fernö M, Fuqua SA, et al. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50:4332–7.
Knight WA, Livingston RB, Gregory EJ, McGuire WL. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977;37:4669–71.
Parkes HC, Lillycrop K, Howell A, Craig RK. C-erbB2 mRNA expression in human breast tumours: comparison with c-erbB2 DNA amplification and correlation with prognosis. Br J Cancer. 1990;61:39–45.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations fort the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16.
Bos R, van Der Hoeven JJ, van Der Wall E, Bos R, van Der Hoeven JJ, van Der Wall E, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–87.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10.
Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48:1266–72.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8.
Buck A, Schirrmeister H, Kühn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23.
Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15:588–93.
Costelloe CM, Rohren EM, Madewell JE, Hamaoka T, Theriault RL, Yu TK, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–14.
Yang WT. Staging of breast cancer with ultrasound. Semin Ultrasound CT MR. 2011;32:331–41.
Higashi T, Tamaki N, Honda T, Torizuka T, Kimura T, Inokuma T, et al. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study. J Nucl Med. 1997;38:1337–44.
Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, et al. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344–8.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer. 2008;112:995–1000.
Straver ME, Aukema TS, Olmos RA, Rutgers EJ, Gilhuijs KG, Schot ME, et al. Feasibility of FDG PET/CT to monitor the response of axillary lymph node metastases to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med. 2010;37:1069–76.
Hodgson NC, Gulenchyn KY. Is there a role for positron emission tomography in breast cancer staging? J Clin Oncol. 2008;26:712–20.
Greco M, Crippa F, Agresti R, Seregni E, Gerali A, Giovanazzi R, et al. Axillary lymph node staging in breast cancer by 2-fluoro-2-deoxy-d-glucose-positron emission tomography: clinical evaluation and alternative management. J Natl Cancer Inst. 2001;93:630–5.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sanli, Y., Kuyumcu, S., Ozkan, Z.G. et al. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med 26, 345–350 (2012). https://doi.org/10.1007/s12149-012-0579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-012-0579-2