Abstract
Purpose
Nivolumab is useful for the treatment of unresectable/recurrent gastric cancer as third-line or later chemotherapy. However, the factors that predict the efficacy of nivolumab monotherapy remain unclear.
Methods
We retrospectively studied the predictive factors of response in 59 consecutive patients treated with nivolumab as third-line or later chemotherapy for unresectable/recurrent gastric cancer at our hospital from October 2017 to May 2020.
Results
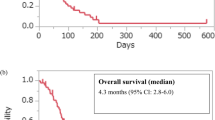
The median follow-up was 5.9 months. The study included 45 men and 14 women (median age: 71 years). We observed that 7 patients had an Eastern Cooperative Oncology Group performance status of 0 and 52 patients had a performance status of 1–2. Forty-three patients were treated with third-line therapy, seven with fourth-line therapy, and three with fifth-line therapy. The response rate to nivolumab was 6.7% and disease control rate was 35.5%. There were 19 (32.2%) immune-related adverse events for all grades and 9 (15.2%) for grades 3 and 4. Progression-free survival was 1.90 months, and overall survival was 6.30 months. Patients with immune-related adverse events had significantly longer overall survival than those without immune-related adverse events. Multivariate analysis showed that the occurrence of immune-related adverse events and a ratio for neutrophil-to-lymphocyte ratio after 8 weeks of nivolumab treatment to the baseline neutrophil-to-lymphocyte ratio before treatment of ≤ 1.5 were independent prognostic factors for overall survival.
Conclusions
Occurrence of immune-related adverse events and changes in neutrophil-to-lymphocyte ratio during nivolumab treatment may help predict the therapeutic efficacy of nivolumab monotherapy for unresectable or recurrent gastric cancer.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Asaka M, Kato M, Graham DY. Prevention of gastric cancer by Helicobacter pylori eradication. Intern Med. 2010;49:633–6.
Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan (Vital Statistics of Japan). Accessed 11 September 2021.
Japanese gastric cancer treatment guidelines 2018 (5th edition).Japanese Gastric Cancer Association. Gastric Cancer. 2021;24:1-21.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–14.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-71.
Chen DS, Mellman I. Oncology meets immunology: the cancer immunity cycle. Immunity. 2013;39:1–10.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–6.
Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–33.
Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87.
Fukui T, Okuma Y, Nakahara Y, Otani S, Igawa S, Katagiri M, et al. Activity of nivolumab and utility of neutrophil-tolymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer. 2019;20:208–14.
Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget. 2018;9:34520–7.
Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol. 2020;85:265–72.
Lalani AA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, et al. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6:5.
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89:1028–30.
Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8:339–45.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–5 (In Japanese).
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32:1200–5.
Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, González P, González B, Mancha A, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20:38–45.
National Cancer Institute. Common terminology criteria for adverse events (CTCAE) ver5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm #ctc_50. Accessed 11 September 2021.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Ribas A, Hersey P, Middleton MR, Gogas H, Flaherty KT, Sondak VK, et al. New challenges in endopoints for drug development in advanced melanoma. Clin Cancer Res. 2012;18:336–41.
Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886–94.
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4:374–8.
Ando T, Ueda A, Ogawa K, Motoo I, Kajiura S, Nakajima T, et al. Prognosis of immune-related adverse events in patients with advanced gastric cancer treated with nivolumab or pembrolizumab: a multicenter retrospective analysis. In Vivo. 2021;35:475–82.
Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. ImmunoTargets Ther. 2017;6:73–82.
Weinmann, S.C. Pisetsky, D.S. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology 2019;58(Suppl. 7):vii59–vii67.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Namikawa T, Munekage E, Munekage M, Maeda H, Yatabe T, Kitagawa H, et al. Evaluation of systematic inflammatory response biomarkers in patients receiving chemotherapy for unresectable and recurrent advanced gastric cancer. Oncology. 2016;90:321–6.
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103.
Toyokawa T, Kubo N, Tamura T, Sakurai K, Amano R, Tanaka H, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer. 2016;16:722.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishido, K., Tanabe, S., Katada, C. et al. Evaluation of Prognostic Factors for Unresectable or Recurrent Gastric Cancer Treated with Nivolumab. J Gastrointest Canc 54, 485–491 (2023). https://doi.org/10.1007/s12029-022-00823-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00823-1