Abstract
This study has analyzed results from registry-based population studies to assess the effect of bariatric surgery upon cancer incidence at a population level. Relevant studies were identified and meta-analysis was used to calculate pooled odds ratios (POR) for the incidence of cancer after bariatric surgery compared to controls. Eight population-based studies were included with 635,642 total patients. Bariatric surgery was associated with a significant reduction in overall cancer incidence (POR = 0.72; 95% CI 0.59 to 0.87; p = 0.0007) and incidence of obesity-related cancer (POR = 0.55; 95% CI 0.31 to 0.96; p = 0.04). Bariatric surgery was also protective for breast cancer development (POR = 0.50; 95% CI 0.25 to 0.99; p = 0.045). Bariatric surgery appears to be associated with a reduction in cancer incidence at a population-based level.
Similar content being viewed by others
Introduction
Obesity is recognized to be an increasing health problem worldwide with 39% of the adult population worldwide being considered overweight or obese [1]. The prevalence of obesity is increasing with 27% of the population of the UK being classified as obese in 2015 (rising from 15% in 1993) [2]. Obesity is an established risk factor for cardiovascular disease, diabetes, and overall all-cause mortality [3,4,5,6]. In more recent years, obesity has also been suggested to be a risk factor for the development of cancer [3, 7,8,9]. Individuals with a body mass index (BMI) above 40 have been identified to have cancer death rates that are significantly higher than that of normal weight individuals (52% higher in men and 62% in women) [10]. In the USA, it has been estimated that in adults over the age of 50 being overweight or obese may account for 14% of cancer deaths in men and 20% in women [10]. Furthermore, it is believed that if the adult population of the USA could maintain a BMI below 25 this may prevent 90,000 cancer deaths per year [10].
Bariatric surgery has been established as the most effective method of achieving sustained weight loss in obese patients. A previous large-scale prospectively matched surgical intervention trial identified weight loss at 10 years in patients receiving bariatric surgery to be between 14 and 25% (dependent upon surgical procedure utilized) compared to a weight change of ± 2% in matched controls [11]. Results from the same trial have also identified that patients undergoing bariatric surgery had a significantly reduced risk of developing cancer (HR 0.67; 95% CI 0.53–0.85; p = 0.0009) [12]. Additional institutional cohort studies have demonstrated that there appears to be a protective effect of bariatric surgery to reduce the risk of cancer development [13,14,15].
Data from clinical trials and large institutional studies may only reflect outcomes in highly specialized centers contributing data to such studies, and fail to fully address the potential benefits of bariatric surgery upon cancer risk, which can be generalized to the obese population at a national level. This systematic review and meta-analysis aimed to assess the influence of bariatric surgery on cancer incidence for obese individuals by analyzing data from large-scale population-based cohort studies.
Methods
A systematic literature search of Medline, EMBASE, and Web of Science was performed. The search terms “bariatric,” “obesity,” ‘cancer,” “malignancy,” and “neoplasm” were utilized along with the medical subject headings (MeSH) “Bariatric Surgery,” “Obesity,” and “Neoplasms.” All search terms were used in combination with the Boolean operators AND or OR. Two authors (T.W. and S.A.) performed the electronic literature search in January 2018. The electronic search was supplemented by a hand-search of published abstracts from relevant specialist conference meetings. Reference lists of all relevant studies were also reviewed to identify potentially relevant studies.
Identified abstracts were independently scrutinized (by T.W and S.A.) to determine eligibility for inclusion. Studies were included if they were registry-based population studies (either at a national or regional level), which reported comparative risk of development of any type of cancer for patients who have undergone any form of bariatric surgery compared to an appropriate control group. Any study which was not a registry-based population study (including randomized controlled trials and institutional studies) was excluded as their results could not be directly applied at a population level. In the situation where two studies utilized the same registry data the study, which reported the most up-to-date data was selected for inclusion, in order to avoid crossover of data. Studies not reported in the English language were excluded.
Data from eligible studies were extracted into a computerized spreadsheet for analysis. Data was collected for overall cancer incidence, obesity-related cancer, and specific cancer types, which were analyzed individually (esophageal, colorectal, breast, endometrial, and prostate cancer).
Statistical analysis was performed using Statsdirect 2.5.7 (Statsdirect Ltd., UK). Pooled outcome measures were determined using random effects models as described by Der Simonian and Laird [16]. Heterogeneity amongst the trials was assessed by Cochran’s Q statistic, a null hypothesis test in which p < 0.05 is taken to indicate the presence of significant heterogeneity and the I2 statistic, which describes the percentage of variation across studies due to significant heterogeneity. The Egger test was used to assess the funnel plot for significant asymmetry, indicating possible publication or other biases. This study was not prospectively registered with any formal registry for systematic reviews.
Results
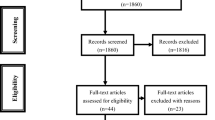
The literature search identified eight population-based studies for inclusion [17,18,19,20,21,22,23,24]. Figure 1 demonstrates the PRISMA flowchart for the literature search. In total, there were 635,642 patients included the analysis. There were 114,020 patients who received bariatric surgery (gastric bypass: 69,740; sleeve gastrectomy: 7519; gastric band: 13,609; gastroplasty: 8632; alternative procedure or undetermined: 14,520). There were 521,622 control patients. The majority of studies utilized patients diagnosed as obese to form the control group [17,18,19, 21, 22, 24]. One study matched patients according to age and gender [20], and one study compared results to the background national population [23]. Table 1 provides the patient demographic details for all studies. Quality assessment of studies was undertaken with the Newcastle Ottawa Scale (Supplementary Table 1) [25]. All studies scored four stars for patient selection (rated out of four stars). Two studies scored one star for comparability as controls did not necessarily have a diagnosis of obesity [20, 23]. All other studies scored the maximum score for comparability (two stars). All studies scored three stars for outcome (rated out of three stars).
Overall Cancer Incidence
Four papers reported overall cancer incidence in patients who had undergone bariatric surgery [17, 19, 20, 24]. Bariatric surgery was associated with a significant reduction in overall cancer incidence compared to control patients (pooled odds ratio (POR) = 0.72; 95% confidence interval (CI) 0.59 to 0.87; p = 0.0007). There was some evidence of statistical heterogeneity (Cochran Q = 11.33; p = 0.01; I2 = 73.5%) but no evidence of bias (Egger = −0.06; p = 0.98) (Fig. 2).
Obesity-Related Cancer
Three studies reported rate of obesity-related cancer defined as cancer of the breast, prostate, colorectum, endometrium, ovary, kidney, esophagus (adenocarcinoma only), liver, pancreas, gallbladder, non-Hodgkin lymphoma, leukemia, and thyroid [17, 23, 24]. Myeloma [17, 24], gastric cardia [24], and meningioma [24] were also included in the definition of obesity-related cancer in some studies (Table 2). An additional study described results for “hormone-related cancers” (including breast, endometrium, and prostate), and these were included as obesity-related cancer for the purposes of analysis [21].
Bariatric surgery was associated with a significant reduction in incidence of obesity-related cancer (POR = 0.55; 95% CI 0.31 to 0.96; p = 0.04). There was some evidence of significant statistical heterogeneity (Cochran Q = 124.34; p = < 0.0001; I2 = 97.6%) but no evidence of bias (Egger = −15.4; p = 0.14) (Fig. 3).
Three studies analyzed rates of obesity-related cancer stratified by gender [21, 23, 24]. When analyzed by gender, it was identified that in male patients bariatric surgery was not associated with a significant reduction in the rate of obesity-related cancer (POR = 0.76; 95% CI 0.87 to 1.32; p = 0.46). However, in female patients who had undergone bariatric surgery, there was a trend towards a reduced rate of obesity-related cancer (POR = 0.50; 95% CI 0.24 to 1.04; p = 0.065) (Supplementary fig. 1). There was evidence of statistical heterogeneity (Cochran Q 1.09.03; p < 0.0001; I2 = 98.2%) and too few strata for an assessment of bias.
Esophageal Cancer
Three studies reported the incidence of esophageal cancer following bariatric surgery [17, 21, 22]. There was no significant effect of bariatric surgery upon the incidence of esophageal cancer (POR = 0.79; 95% CI 0.43 to 1.44; p = 0.43). There was no evidence of statistical heterogeneity (Cochran Q = 0.74; p = 0.69; I2 = 0%). There were too few strata to facilitate an analysis of bias.
Colorectal Cancer
Four studies reported the incidence of colorectal cancer [17, 18, 21, 23]. Bariatric surgery was not associated with a statistically significant change in the incidence of colorectal cancer (POR = 1.39; 95% CI 0.96 to 2.02; p = 0.08). There was some evidence of statistical heterogeneity (Cochran Q = 10.99; p = 0.01; I2 = 72.7%) but no evidence of bias (Egger = − 1.43; p = 0.71) (Supplementary fig. 2).
Breast Cancer
Three studies reported the rate of breast cancer in patients who have undergone bariatric surgery [17, 21, 23]. Bariatric surgery was associated with a significant reduction in the incidence breast cancer compared to controls (POR = 0.50; 95% CI 0.25 to 0.99; p = 0.045) (Fig. 4). There was some evidence of statistical heterogeneity (Cochran Q = 39.4; p < 0.0001; I2 = 94.9%). There were too few strata to facilitate an analysis of bias.
Endometrial Cancer
Three studies reported the rate of endometrial cancer in bariatric surgery patients [17, 21, 23]. There was no significant difference in the rate of endometrial cancer between patients who had undergone bariatric surgery and controls (POR = 0.47; 95% CI 0.08 to 2.65; p = 0.39). There was some evidence of statistical heterogeneity (Cochran Q = 94.8; p = < 0.0001; I2 = 97.9%). There were too few strata to facilitate an analysis of bias.
Prostate Cancer
Three studies reported the rate of prostate cancer in these patients [17, 21, 23]. Bariatric surgery was not associated with any significant differences in the incidence of prostate cancer (POR = 0.82; 95% CI 0.39 to 1.73; p = 0.61). There was evidence of statistical heterogeneity (Cochran Q = 8.86; p = 0.01; I2 = 77.4%). There were too few strata to facilitate an analysis of bias.
Discussion
This study has established that bariatric surgery appears to be associated with a reduced incidence of cancer at a population level. Overall cancer incidence (POR = 0.72; 95% CI 0.59 to 0.87; p = 0.0007) and incidence of obesity-related cancers (POR = 0.55; 95% CI 0.31 to 0.96; p = 0.04) were both significantly reduced in patients who had undergone bariatric surgery. When analyzing specific cancer types bariatric surgery only appeared to have a significant protective effect in breast cancer (POR = 0.50; 95% CI 0.25 to 0.99; p = 0.045) (Table 3).
The results of this meta-analysis of population-based studies are consistent with those reported from previous large-scale clinical trials. Previous studies have demonstrated a reduction in cancer incidence following bariatric surgery with the greatest protection being provided to women [12], with a marked reduction in breast cancer risk [13]. These results appear to have been reproduced here with a trend towards reduced obesity-related cancer in women undergoing bariatric surgery and a significant reduction in breast cancer risk.
The mechanisms responsible for the reduction in cancer incidence associated with bariatric surgery are believed to be multifactoral [26]. This effect is believed to be related to reduced systemic inflammation and oxidative stress, as well as the influence of surgery upon insulin resistance, sex steroids, gut hormones, and adipokines [26, 27]. Although most previous studies have identified a reduced incidence of cancer following bariatric surgery, gastric bypass has been associated with increased risk of colorectal cancer [18, 21, 23]. It has been hypothesized that this effect may be due to the increased presence of bile acids within the colon following gastric bypass [28], and persistent abnormalities in rectal mucosa have been identified in patients undergoing this procedure [29]. The association between bariatric surgery and increased risk of colorectal cancer was not reproduced in the current meta-analysis, although it may be suggested that there was a trend towards a slight increased risk of colorectal cancer development (POR = 1.39; 95% CI 0.96 to 2.02; p = 0.08). Although the current analysis did include patients who underwent procedures other than gastric bypass, of the four studies [17, 18, 21, 23] which provided data regarding the incidence of colorectal cancer following surgery only the study by Adams et al. demonstrated a protective effect of bariatric surgery upon colorectal cancer risk [17]. This study exclusively included patients undergoing gastric bypass; therefore, the underlying cause for this discrepancy in results is unclear. Further research is required to accurately define the risk and mechanism for colorectal cancer development following gastric bypass surgery and potential need for colonoscopy screening in these patients.
There are important limitations inherent with this type of pooled analysis that must be taken into account when interpreting the results present here. All the included studies were large-scale comparative studies from registry-based data, and none were randomized in nature. It is therefore difficult to account for potential important confounding variables, which may not have been equally distributed between patient groups. However, large-scale registry-based studies were deliberately selected to be included in the current analysis in order to identify how bariatric surgery may influence cancer incidence at a population level. Most studies took account of specific patient factors in order to match groups as far as is feasible. One important factor which was not available in some studies was BMI data for included patients. BMI was only reported in three studies [17, 19, 24], although other studies took measures to only include those patients in the control group who had been diagnosed with “obesity.” Only one study compared outcomes to data from the overall background population [23], and another study matched patients in the control group based upon age and gender [20]. A further limitation is that for around 13% of bariatric surgery cases (n = 14,520) the specific surgical procedure was undetermined. This was largely influenced by procedure type being unreported for the patients who did not go on to develop cancer in one study [23]. However, no specific sub-group analysis was performed by procedure type, and this should not have influenced results. Although previous meta-analyses have demonstrated a reduced incidence of cancer following bariatric surgery [30, 31], the current study is the first of this form of analysis to purely utilize population-based studies, and includes data from a much greater number of individual patients (total of 635,642 patients). This study therefore provides a greater insight into the effects of bariatric surgery at a national level in terms of cancer incidence.
In conclusion, this meta-analysis has demonstrated that at a national level bariatric surgery is associated with a reduction in overall cancer incidence, with a specific reduction in the incidence of obesity-related cancers and breast cancer. This finding may have implications when counseling patients considering bariatric surgery and also when considering the potential effects of bariatric surgery from a public health and commissioning perspective. Further research is necessary to precisely delineate the underlying mechanism responsible for the cancer-protective effects of bariatric surgery.
References
WHO | Overweight and obesity. WHO. World Health Organization; 2017. Available from: http://www.who.int/gho/ncd/risk_factors/overweight/en/. Last accessed 08/10/2018.
NHS Digital. Statistics on Obesity, Physical Activity and Diet. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/613532/obes-phys-acti-diet-eng-2017-rep.pdf. Last accessed 08/10/2018.
Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359(20):2105–20.
Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet (London, England). Elsevier. 2009;373(9669):1083–96.
Zhang C, Rexrode KM, Van Dam RM, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117(13):1658–67.
Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14.
Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78.
Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:j477.
Steele CB, Thomas CC, Henley SJ, et al. Vital signs: trends in incidence of cancers associated with overweight and obesity — United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052–8.
Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–383.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–52.
Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10(7):653–62.
Christou NV, Lieberman M, Sampalis F, et al. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. American Society for Metabolic and Bariatric Surgery. 2008;4(6):691–5.
McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: potential protective impact of bariatric surgery. J Am Coll Surg. American College of Surgeons. 2009;208(6):1093–8.
Ward KK, Roncancio AM, Shah NR, et al. Bariatric surgery decreases the risk of uterine malignancy. Gynecol Oncol. 2014;133(1):63–6.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity. 2010;17(4):796–802.
Derogar M, Hull MA, Kant P, et al. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013;258(6):983–8.
Douglas IJ, Bhaskaran K, Batterham RL, et al. Bariatric surgery in the United Kingdom: a cohort study of weight loss and clinical outcomes in routine clinical care. PLoS Med. 2015;12(12):e1001925.
Gribsholt SB, Thomsen RW, Svensson E, et al. Overall and cause-specific mortality after Roux-en-Y gastric bypass surgery: a nationwide cohort study. Surg Obes Relat Dis. 2017;13(4):581–7.
Mackenzie H, Markar S, Askari A, Faiz O, Hull M, Purkayastha S, et al. Obesity surgery and cancer risk: an English population-based cohort study. Br J Surg. 2018.
Maret-Ouda J, Tao W, Mattsson F, et al. Esophageal adenocarcinoma after obesity surgery in a population-based cohort study. Surg Obes Relat Dis. 2017;13(1):28–34.
Östlund MP, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg. 2010;252(6):972–6.
Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2017;21:1.
Wells GA, Shea B, O’Connell D, Paterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Last accessed 08/10/2018.
Ashrafian H, Ahmed K, Rowland SP, et al. Metabolic surgery and cancer. Cancer. 2011;117(9):1788–99.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–83.
Penney NC, Kinross J, Newton RC, et al. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes. 2015;39(11):1565–74.
Kant P, Sainsbury A, Reed KR, et al. Rectal epithelial cell mitosis and expression of macrophage migration inhibitory factor are increased 3 years after Roux-en-Y gastric bypass (RYGB) for morbid obesity: implications for long-term neoplastic risk following RYGB. Gut. 2011;60(7):893–901.
Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26(11):2590–601.
Casagrande DS, Rosa DD, Umpierre D, et al. Incidence of cancer following bariatric surgery: systematic review and meta-analysis. Obes Surg. 2014;24(9):1499–509.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
For this type of study, formal consent is not required.
Informed Consent Statement
Does not apply.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary figure 1
(DOCX 14 kb)
Supplementary figure 2
(DOCX 14 kb)
Supplementary table 1
(DOCX 22 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wiggins, T., Antonowicz, S.S. & Markar, S.R. Cancer Risk Following Bariatric Surgery—Systematic Review and Meta-analysis of National Population-Based Cohort Studies. OBES SURG 29, 1031–1039 (2019). https://doi.org/10.1007/s11695-018-3501-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3501-8