Abstract
Background
Although olaparib, the first poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor approved, has been used in routine clinical practice for over three years, little has been published on its uptake, utilization patterns, and clinical outcomes.
Objective
To examine real-world use and outcomes of olaparib treatment in Swedish patients during the first three years following regulatory approval.
Patients and Methods
This is a population-based cohort study using data from the Swedish national registers. All individuals initiating olaparib treatment from regulatory approval to 31 December 2017 were included. The extent of off-label use was assessed based on recorded diagnoses. Ovarian cancer patients were followed until death or the end of the study period. Starting dose and dose adjustments were assessed. Time to olaparib discontinuation and overall survival were plotted using Kaplan–Meier survival curves.
Results
We identified 109 patients to whom olaparib was dispensed in Sweden during the study period. Nine of these were prescribed olaparib off-label for either breast or prostate cancer and were excluded from further analyses. Median age among the remaining 100 patients with ovarian cancer was 59 years (range: 42–83). Almost all patients (96%) started on the recommended dose (400 mg [eight capsules] taken twice daily). Dose reductions were explicitly recorded for 14% of patients. Median time to discontinuation was 289 days (95% confidence interval [CI]: 226; 338). Median overall survival from olaparib initiation was 1002 days (95% CI: 676; not calculable).
Conclusions
To our knowledge, this is the first population-based study of olaparib real-world use and outcomes. During the first three years following regulatory approval, olaparib was mainly prescribed to ovarian cancer patients. Ovarian cancer patients stayed on olaparib for a median of 9.5 months and the treatment appeared to be well tolerated.
Similar content being viewed by others
In the first three years following regulatory approval, over 100 patients were treated with olaparib in Sweden. Olaparib was mainly prescribed to ovarian cancer patients who stayed on treatment for a median of 9.5 months. | |
In addition to its use in ovarian cancer, olaparib was also adopted off-label, particularly in breast cancer, even though evidence supporting such use was still limited at the time. | |
Policies and tools to facilitate access to data collected in electronic health records are needed to enable a more comprehensive assessment of new cancer drugs. |
1 Introduction
The oncology research and development pipeline has seen a steady growth over the past 20 years with scientific advances paving the way for innovative approaches to treat cancer. Targeted drugs, including small molecule inhibitors and biologicals targeting oncogenic pathways and immune checkpoints, are now estimated to account for over 90% of oncology drugs in late phase clinical development [1]. Of drugs targeting DNA repair, poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors have been studied most extensively [2]. To date, four PARP inhibitors—olaparib (Lynparza), niraparib (Zejula), rucaparib (Rubraca), and most recently, talazoparib (Talzenna)—have received regulatory approval.
Olaparib was the first PARP inhibitor to come to market. It was approved in 2014 by the European Medicines Agency (EMA) as a capsule formulation for women with BRCA-mutated platinum-sensitive relapsed high grade serous ovarian cancer; this approval was based on the results from a phase II study (Study 19) [3, 4]. In 2018, based on results from SOLO-2 [5], EMA approved a new tablet formulation of olaparib, making the drug available for a broader group of women with platinum-sensitive relapsed high grade ovarian cancer regardless of BRCA status. Also, a marketing authorization application for the use of olaparib tablets in patients with BRCA-mutated, HER2-negative metastatic breast cancer was recently submitted to EMA [6]. In the United States, in addition to ovarian cancer, olaparib has been approved to treat germline BRCA-mutated metastatic breast cancer (based on the OlympiAD study) [7, 8]. Also, in the SOLO-1 trial olaparib demonstrated a significant and clinically meaningful improvement in reducing the risk of progression for newly-diagnosed patients with advanced BRCA-mutated ovarian cancer following platinum-based chemotherapy, [9] and it is expected that approval from regulatory authorities will now be sought to expand the use of olaparib to these patients [10]. Moreover, olaparib is currently being tested in a range of tumor types in addition to ovarian and breast, including prostate and pancreatic cancers [11]. Finally, PARP inhibitors, including olaparib, may even hold therapeutic potential in non-oncological diseases [12].
Although olaparib has been used in routine clinical practice for over three years, little has been published on its uptake, utilization patterns, and clinical outcomes. However, timely analyses of accumulated real-world data are necessary to inform stakeholders, including patients, clinicians, payers, and regulators [13,14,15]. It also is of general interest to explore whether already established data sources can be used to generate real-world evidence on new cancer drugs. The aim of this study was to describe patient characteristics, assess the extent of off-label use, review dose interruptions, dose reductions, and use of concomitant medicinal products for managing side effects, and assess time to discontinuation and overall survival in patients treated with olaparib during the first three years following regulatory approval.
2 Methods
2.1 Study Design
This is a population-based cohort study of all individuals residing in Sweden who were treated with olaparib from regulatory approval to 31 December 2017. The study was approved by the regional ethics board in Stockholm, Sweden (ref. no. 2012–1236-31-4; amendment no. 2015–1790-32). Informed consent was not obtained (and not required by the ethics board) because the data were anonymized before we were given access to them.
2.2 Data Sources
All data used in this study were obtained from the Swedish national population-based registers [16, 17]. Data from the registers were linked using a unique personal identification number. The data were provided by the Swedish National Board of Health and Welfare and Statistics Sweden in April 2018 and included all information recorded from 1 January 2005 to 31 December 2017 (data from the Cancer Register were only available until 31 December 2016).
We used the National Patient Register to obtain information on diagnoses (International Classification of Diseases [ICD]-10) and procedures (Swedish Classification of Health Interventions and the Nordic Medico-Statistical Committee [NOMESCO] codes) recorded in inpatient and outpatient specialist care. Outpatient drug utilization records (dispensations of prescribed drugs) were derived from the Prescribed Drug Register. The Cancer Register was used to retrieve information on primary cancers that in Sweden are mandatorily reported upon detection, including information on the primary site of the tumor, its malignancy, histology, stage, and the date of diagnosis. Mortality data were derived from the Causes of Death Register. Data on migration were retrieved from the Total Population Register.
Moreover, we also obtained aggregate monthly data on hospital sales of olaparib from 1 December 2014 to 31 December 2017 to estimate use of olaparib not captured at the individual level. These data were provided by the eHealth Agency that records complete pharmaceutical sales data from all pharmacies, retailers, and wholesalers in Sweden [18].
2.3 Study Population
All patients in Sweden who were dispensed olaparib (ATC code: L01XX46) from regulatory approval to 31 December 2017 were included in the study population. The index date was defined as the date of the first olaparib dispensation for each patient.
Patients’ data were available as far back as 1 January 2005 (the starting date for the data cut used in this study). Patients were followed from the index date until the earliest of the following: emigration, death, or the end of the study period (31 December 2017).
2.4 Assessment of Clinical Indications and Off-Label Use
During the study period olaparib was approved in the European Union for use as monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed BRCA-mutated ovarian (including fallopian tube or primary peritoneal) cancer who are in response (complete or partial) to platinum-based chemotherapy. In parallel, evidence from clinical trials was emerging in support of olaparib use in a broader group of ovarian cancer patients regardless of BRCA status [5], as well as in breast cancer [7]. To determine the indication that olaparib was prescribed for, we reviewed diagnoses and procedures recorded in the National Patient Register as well as data reported to the Cancer Register. Moreover, free-text documentation on directions for use in the Prescribed Drug Register was reviewed for information on the indication for which the drug was prescribed.
To quantify the use of olaparib in patients with ovarian (including fallopian tube and peritoneal) cancer, we first reviewed data reported to the Cancer Register to identify all patients with primary ovarian (C56.9), fallopian tube (C57.0, C57.9), or peritoneal (C48.2) cancer. Moreover, patients with diagnoses of ovarian, fallopian tube, or peritoneal cancer recorded in the National Patient Register were also classified as treated for ovarian cancer. Finally, if the directions for use information in the Prescribed Drug Register specified that olaparib was prescribed for ovarian cancer, and the recorded diagnoses and procedures were in line with this, the use of olaparib was also attributed to the treatment of ovarian cancer.
We then explored whether data from the Swedish national registers would allow us to discern if olaparib was used in BRCA-positive and platinum-sensitive ovarian cancer patients. Various proxies for being BRCA-positive were explored, including procedure codes related to genetic testing (AV070, AV071, DV026, ZV017, ZV018), comorbid breast cancer diagnosis (C50*; Z85.3), and family history of breast (Z80.3) and/or gynecological (Z80.4) cancer. Proxies for prior platinum-based therapy were based on diagnostic (Z08.2, Z51.1) and procedure (DT008, DT016, DT026, DT108, DT116) codes as well as ATC codes (L01XA**) recorded in the National Patient Register.
The use of olaparib for other cancers was considered off-label. Assessment of olaparib use and treatment outcomes, as described below, was restricted to patients with ovarian cancer.
2.5 Assessment of Olaparib Use
Data on olaparib dispensations were derived from the Prescribed Drug Register. Only one olaparib product was available on the Swedish market during the study period (hard capsules, 50 mg; dispensed in a pack of 448 capsules [4 bottles of 112 capsules]). We retrieved information on olaparib prescription and dispensation dates, amount dispensed, and directions for use as prescribed. The directions for use variable is a free text variable that typically contains instructions on how to take the prescribed drug as specified by the prescriber; for example, “400 mg (eight capsules) twice a day”.
We assessed the prescribed starting dose as well as dose adjustments made throughout the course of olaparib treatment. We assumed that the date when a new prescription with new directions for use was issued would reflect the date from which the patient would be advised to adjust the dosing regimen and that any remaining supply from the previous prescription would be used in line with the new directions for use starting on this date.
Duration of olaparib exposure was estimated using the dispensation date together with the number of dispensed packages and the directions for use. Based on an assumption that patients fully adhered to the prescribed regimen, we flagged intervals without olaparib supply as possible dose interruptions. However, considering that some patients may have lowered the dose without this being reflected in the records, we also explored an alternative scenario in which intervals without olaparib supply could also be explained by dose reductions (as the dispensed supply taken at a lower dose would have lasted for a longer period).
We also described utilization of metoclopramide (A03FA01) and ondansetron (A04AA01) that are typically used in Sweden to manage nausea and vomiting in cancer patients.
2.6 Assessment of Olaparib Treatment Outcomes
We assessed the following treatment outcomes: time to olaparib discontinuation and overall survival. Time to olaparib discontinuation was defined as time from the index date to the end of supply of dispensed olaparib or death. The end of olaparib supply was considered to be a discontinuation if it was followed by a number of days equaling the sum of the amount of supply dispensed at the date of the last dispensation and any stockpiled supply remaining at the date with no new dispensations recorded. Overall survival was defined as time from the index date to the date of death from any cause. Patients remaining on olaparib at the end of the study period were censored.
2.7 Statistical Analyses
Descriptive statistics were used to summarize data on baseline characteristics of the study population. For categorical variables, we reported frequencies and proportions. For continuous variables, we reported the median and range. Time to olaparib discontinuation and overall survival were plotted using Kaplan–Meier curves. Data management and analyses were conducted using SAS 9.4 (Cary, NC).
3 Results
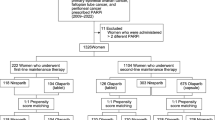
We identified 109 patients to whom olaparib was dispensed during the study period (in total 858 packs dispensed). Our review of the aggregate monthly sales data showed that an additional 105 packs, which could not be attributed to individual patients, were purchased by hospitals during the same period. No use was recorded prior to the date of olaparib inclusion in the pharmaceutical benefits scheme (25 February 2015).
All patients had at least two years of continuous residence in Sweden prior to the index date and almost all (95%) resided in Sweden from 1 January 2005 or earlier. None of the study patients emigrated during the follow-up period. Thus, we were able to follow all patients until either death or the end of the study period (31 December 2017). The median follow-up time among all patients was 319 days (range: 10–1038).
3.1 Clinical Indications and Off-Label Use
Among the 109 patients, 89 had ovarian, fallopian tube, or peritoneal cancer reported to the Cancer Register, with the majority diagnosed at advanced stages (FIGO III or IV). Median time from cancer being recorded in the Cancer Register to the first dispensation of olaparib was three years and one month (ranging from one year and four months to over 12 years). Moreover, an additional eleven patients had diagnoses of ovarian, fallopian tube, or peritoneal cancer recorded in the National Patient Register. The remaining nine patients were prescribed olaparib for either breast or prostate cancer. In total, 100 patients were assumed to be treated for ovarian cancer based on the recorded data. The median follow-up time among the ovarian cancer patients was 396 days (range: 31–1038). Baseline characteristics of these patients are presented in Table 1.
Procedure codes related to genetic testing were found in the records of 28 patients. Of these, 19 had procedure codes indicating possible BRCA1/2 testing prior to olaparib therapy (timing ranged from three months to almost eight years prior to olaparib). Twenty-eight patients (28%) were diagnosed with breast cancer prior to ovarian cancer. Family history of breast cancer, gynecological cancer, or both was recorded in 53%, 27%, and 25% of patients, respectively. Of the 28 patients with recorded procedure codes related to genetic testing, 16 had a personal history of breast cancer and 26 had recorded family history of either breast or gynecological cancer (visualization of these data is available in the electronic supplementary material).
Diagnostic and procedure codes related to chemotherapy were recorded in 75% and 93% of patients, respectively. In total, 96 had either diagnostic or procedure codes suggesting administration of chemotherapy. Of these, only 32 patients had a record specifying that they had been treated with platinum-based chemotherapy prior to being dispensed olaparib. The timing of the most recent platinum-based chemotherapy record before olaparib ranged from one month to almost six years.
3.2 Dose Adjustments
At the time of olaparib initiation, the majority of ovarian cancer patients (96 out of 100) started on the recommended dose of 400 mg (eight capsules) taken twice daily. A new prescription for a reduced dose was issued to 14 patients (14%) after a median of 78 days (range: 20–344). In addition, we observed dispensation patterns indicative of possible dose reductions in at least 13 more patients. Overall, 59 patients had possible intervals without olaparib supply that ranged from one to 74 days. Finally, during the course of olaparib treatment, 36 patients were dispensed either metoclopramide or ondansetron.
3.3 Time to Treatment Discontinuation and Overall Survival
Fifty-seven patients discontinued olaparib during the follow-up period. Median time to discontinuation was 289 days (95% confidence interval [CI]: 226; 338) (Fig. 1). Among patients who had more than one year of follow-up data, 44% were receiving olaparib for at least one year. Among patients who had more than two years of follow-up data, 33% were receiving olaparib for at least two years.
Twenty-six patients died during the follow-up period, with the majority of these being off olaparib treatment at the time of death. Median overall survival from olaparib initiation was 1002 days (95% CI: 676; not calculable) (Fig. 2).
4 Discussion
In the first three years following its regulatory approval in December 2014, olaparib was used in 100 ovarian cancer patients in Sweden (all receiving cancer care, directly or indirectly, at university hospitals). Median time to olaparib discontinuation was 9.5 months. Dose reductions were explicitly recorded in 14 patients (only five of these would discontinue olaparib right after reducing the dose) and dispensation patterns indicative of possible dose reductions were observed in at least 13 more patients (combined, at least 27% had to either reduce the dose or interrupt treatment). Most patients continued on olaparib after dose reductions or treatment interruptions. In addition to its use in ovarian cancer, olaparib was also prescribed off-label to a small number of breast or prostate cancer patients.
To our knowledge, this is the first population-based study of olaparib real-world use and outcomes. Other real-world data available so far come from manufacturer-funded studies, to date presented only as conference abstracts. A study of French patients who received olaparib through a compassionate use programme showed that olaparib was well tolerated [19]. Interim results of the non-interventional C-PATROL study in Germany [20] indicated that while it was common for patients to experience adverse events—particularly anemia, nausea, and fatigue—the toxicity of olaparib was manageable and the drug was well tolerated [21]. There is also an ongoing phase IV study of olaparib effectiveness and safety (ORZORA), however no results have yet been presented [22, 23]. Our study is different from C-PATROL and ORZORA in terms of the questions addressed (assessment of olaparib use in routine clinical practice versus assessment of effectiveness and safety of olaparib when used according to the approved indication), data sources used (secondary data versus primary data collection), and outcomes measured (e.g. time to treatment discontinuation versus progression free survival [PFS]).
Using data from the Swedish national registers, we were able to measure time to treatment discontinuation and overall survival. These endpoints were studied as secondary outcomes in Study 19 as well as in C-PATROL and ORZORA. While we explored the possibility of estimating real-world PFS that would correspond to the primary endpoint in the pivotal trial and in the other real-world studies, it became clear that the data routinely recorded in the Swedish national registers are not sufficient for this purpose.
Compared to the median time to olaparib discontinuation among BRCA-positive patients reported in Study 19 (11 months) [24], Swedish patients discontinued olaparib treatment earlier (9.5 months). However, in Study 19 patients were allowed to continue treatment even after documented progression, but such practice is not in line with the approved indication and it is unlikely that our study patients would have continued olaparib treatment after progression. While time to discontinuation is a composite endpoint that comprises discontinuations for any reason, it can nonetheless be considered a possible proxy for PFS, particularly if patients mainly discontinue due to progression. While we were not able to determine the reasons for discontinuation, the only real-world study so far reporting such data (C-PATROL) showed that most patients (82%) stopped olaparib due to progression, with few discontinuing due to an adverse event [21]. It may be that disease progression was the dominant reason leading to discontinuation in our study patients too. If considering our estimate as a proxy it should be kept in mind that it may both underestimate and overestimate real-world PFS. For example, the former would occur in patients who stopped treatment for any reason other than progression prior to actual progression; the latter would be seen in patients who progressed while still having stockpiled olaparib (in general, it is unlikely that patients who progress do so on the very last day of their dispensed supply).
Our estimate of median overall survival is similar to what was reported in Study 19 (33 versus 35 months). Only a few patients died while on olaparib treatment and the majority exhausted their dispensed olaparib supply prior to their death, possibly receiving subsequent chemotherapy during the post-olaparib period. Given that a large number of patients (74%) were alive at the end of the study, our overall survival estimate should be interpreted with caution.
In addition to the above discussed endpoints, our study assessed how olaparib was used in Swedish routine clinical practice in the first years on the market. The characteristics of ovarian cancer patients that we were able to derive were similar to those of the patients included in Study 19. We saw that olaparib was also adopted off-label, particularly in breast cancer, even though evidence supporting such use was still limited at the time. Moreover, the uptake in ovarian cancer may have been somewhat slower than expected. At the time of the olaparib introduction in Sweden it was anticipated that 50 to 75 patients per year would be treated (equating to 150 to 225 accrued in the first three years on market) [25] compared to 100 patients with ovarian cancer who actually were dispensed olaparib. One possible explanation for this discrepancy is that routines for BRCA1/2 genetic testing may have been insufficient to identify all patients who would be candidates for olaparib treatment.
The strengths and weaknesses of our study primarily relate to the data sources we used. We included all patients dispensed olaparib in Sweden. This allowed estimating the extent of off-label olaparib use. Moreover, we were able to follow up all patients until either death or the end of the study period. Linkage to high-quality death records [26] made it possible to estimate overall survival.
Data on dispensations of prescribed drugs from the Prescribed Drug Register [27], which have been extensively used in research [28], allowed us to review dose adjustments made during the course of olaparib treatment, to assess concomitant use of medicines to manage side effects, and, importantly, to estimate time to olaparib discontinuation. These data, however, cover only prescribed drugs dispensed in pharmacies. While our review of sales data did show that some in-hospital use of olaparib occurred during the study period, the dispensation data nonetheless captured 89% of all olaparib use in Sweden. Also, dose adjustments could be reliably captured only if a new prescription with new directions for use was issued. Our exploratory analyses based on a review of the entire dispensation pattern suggest that it is likely that around one third of the study patients had to either reduce the dose or interrupt treatment. Also, no data on reasons for discontinuation were available, leaving a gap in our understanding regarding why exactly patients discontinue treatment. Likewise, no information on reasons for dose reductions was available either.
Despite the obvious richness of data collected in the Swedish national registers, clinical information is scarce. While the Cancer Register does record extended information on reported cancers (e.g. histological type, stage, and basis for diagnosis) [29], most of the variables in the National Patient Register are of administrative nature. However, diagnosis and procedure codes allowed us to explore patient interaction with the healthcare system [30]. We found, though, that despite the existence of these fields that could be used to capture data on administered chemotherapy (including the possibility to specify ATC codes), information on chemotherapy was either inconsistently recorded or missing completely. Similarly, despite the existence of procedure codes for genetic testing and genetic counseling, less than one third of patients had such codes present in their records, most likely explained by incomplete recording (as there was no specific incentive to record these data) rather than the absence of services provided (national guidelines in Sweden recommend genetic testing, which is publicly funded, for all patients with high grade serous ovarian cancer [31]). While a record of a procedure code for genetic testing would still not specify BRCA status, such information could still be of interest (e.g. it would be expected that all patients treated with olaparib had been tested prior to treatment). Other relevant clinical information, including performance status, time to progression on penultimate platinum therapy, and response to the most recent platinum therapy prior to olaparib initiation, was not available to us, making it difficult both to fully assess whether olaparib was used according to the approved indication and to compare the patients included in our study to those who participated in Study 19. It is possible, for example, that ovarian cancer patients receiving olaparib in Swedish routine clinical practice had worse performance status compared with the participants in Study 19. Lack of information on BRCA status and chemotherapy administered in hospitals also prevented us from identifying all ovarian cancer patients potentially eligible for olaparib treatment and studying if all who would benefit from olaparib received treatment.
Additionally, it is possible that we both underestimate and overestimate the duration of olaparib treatment. Time to olaparib discontinuation would be underestimated in patients who were on a lower dose than specified in the directions for use of the dispensed prescription and overestimated in patients who stopped treatment before exhausting the supply dispensed to them.
Finally, our study population is rather small, which is, however, expected for an orphan medicine (during the study period olaparib had orphan designation). All patients dispensed olaparib in Sweden were included with the total number reaching 100 (in Study 19 there were 136 patients assigned to olaparib). Lack of data maturity creates uncertainty in our estimates, particularly for overall survival. In general, this highlights the importance of balancing the desire to have information on real-world use and, in particular, treatment outcomes early on after the introduction of a new drug, with allowing for sufficient follow-up time to accrue.
It is possible that some of the above-mentioned limitations could have been addressed if there was access to data from electronic health records (EHRs). There are encouraging examples of the use of EHRs in the follow-up of new cancer drugs. For example, studies in the United States showed the possibility of estimating real-world PFS based on EHR data [32, 33]. In Sweden EHRs have been implemented nationally since 2012, but access to these data, particularly at the national level, is not straightforward [34]. Studies in other therapeutic areas done at a regional level, however, have already demonstrated that EHRs enrich data recorded in the Swedish national registers to enable a broader range of research questions to be addressed [35,36,37].
To summarize, this is the first population-based study of olaparib real-world use and outcomes. In the first three years following regulatory approval, olaparib was mainly prescribed to ovarian cancer patients who stayed on treatment for a median of 9.5 months. The treatment appeared to be well tolerated. More broadly, this study also highlights both opportunities and challenges in the assessment of real-world use and outcomes of new cancer drugs.
References
IQVIA Institute for Human Data Science. Global Oncology Trends 2018: Innovation, Expansion and Disruption. 2018.
Gavande NS, VanderVere-Carozza PS, Hinshaw HD, Jalal SI, Sears CR, Pawelczak KS, et al. DNA repair targeted therapy: the past or future of cancer treatment? Pharmacol Ther. 2016;160:65–83. https://doi.org/10.1016/j.pharmthera.2016.02.003.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61. https://doi.org/10.1016/s1470-2045(14)70228-1.
Deeks ED. Olaparib: first global approval. Drugs. 2015;75(2):231–40. https://doi.org/10.1007/s40265-015-0345-6.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84. https://doi.org/10.1016/s1470-2045(17)30469-2.
AstraZeneca. The European Medicines Agency accepts regulatory submission for Lynparza in BRCA-mutated HER2-negative metastatic breast cancer [press release]. https://www.astrazeneca.com/media-centre/press-releases/2018/the-european-medicines-agency-accepts-regulatory-submission-for-lynparza-in-brca-mutated-her2-negative-metastatic-breast-cancer-03042018.html. 2018.
Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–33. https://doi.org/10.1056/NEJMoa1706450.
First PARP Inhibitor Ok’d for Breast Cancer. Cancer Discov. 2018;8(3):256–7. https://doi.org/10.1158/2159-8290.cd-nb2018-008.
Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance Olaparib in patients with newly diagnosed advanced ovarian Cancer. N Engl J Med. 2018. https://doi.org/10.1056/NEJMoa1810858.
AstraZeneca. SOLO-1 Phase III trial demonstrates Lynparza maintenance therapy cut risk of disease progression or death by 70% in patients with newly-diagnosed, advanced BRCA-mutated ovarian cancer [press release]. https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2018/solo-1-phase-III-trial-demonstrates-lynparza-maintenance-therapy-cut-risk-of-disease-progression-or-death-by-70-percent-in-patients-with-newly-diagnosed-advanced-brca-mutated-ovarian-cancer.html. 2018.
Du Y, Yamaguchi H, Hsu JL, Hung M-C. PARP inhibitors as precision medicine for cancer treatment. Ntnl Sci Rev. 2017;4(4):576–92. https://doi.org/10.1093/nsr/nwx027.
Berger NA, Besson VC, Boulares AH, Burkle A, Chiarugi A, Clark RS, et al. Opportunities for the repurposing of PARP inhibitors for the therapy of non-oncological diseases. Br J Pharmacol. 2018;175(2):192–222. https://doi.org/10.1111/bph.13748.
Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii61–5. https://doi.org/10.1093/annonc/mdx443.
Jonsson B. Bringing in health technology assessment and cost-effectiveness considerations at an early stage of drug development. Mol Oncol. 2015;9(5):1025–33. https://doi.org/10.1016/j.molonc.2014.10.009.
Khozin S, Blumenthal GM, Pazdur R. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109(11). https://doi.org/10.1093/jnci/djx187.
Statistics Sweden. www.scb.se. 2018.
The National Board of Health and Welfare. www.socialstyrelsen.se. 2018.
Swedish eHealth Agency. www.ehalsomyndigheten.se. 2018.
De La Motte Rouge T, Pautier P, Alexandre J, Ray-Coquard I, Cottu P, Rodrigues M, et al. 2755 first real life data on Olaparib in BRCA1/2 mutated platinum-sensitive relapsed (PSR) epithelial ovarian cancer (EOC) in France: analysis of 52 patients (pts) enrolled in the French temporary authorization for use (ATU). Eur J Cancer. 2015;51:S548–9. https://doi.org/10.1016/S0959-8049(16)31521-0.
AstraZeneca. C-PATROL - Non-interventional Study (NIS) to Collect Clinical and Patient Reported Outcome Data in an Olaparib Treated BRCAm+ PSR Ovarian Cancer Population. www.clinicaltrials.gov/show/NCT02503436. 2018.
Marme F, Hilpert F, Welslau M, El-Balat A, Grischke E-M, Schinkothe T, et al. Olaparib in German routine clinical practice: updated interim results of the non-interventional study C-PATROL. J Clin Oncol. 2018;36(15_suppl):e17549. https://doi.org/10.1200/JCO.2018.36.15_suppl.e17549.
Pignata S, Lewis J, Tchakov I, Robertson JD, Morris T, Jayawardene D, et al. ORZORA: open-label phase IV trial of olaparib in patients with BRCA-mutated ovarian cancer. Int J Gynecol Cancer. 2015;25(Suppl 2):1469. https://doi.org/10.1097/01.IGC.0000473498.85773.6e.
AstraZeneca. To Assess the Efficacy and Safety of Olaparib Maintenance Monotherapy in the Treatment of Ovarian Cancer (ORZORA). www.clinicaltrials.gov/show/NCT02476968. 2015.
Hettle R, Posnett J, Borrill J. Challenges in economic modeling of anticancer therapies: an example of modeling the survival benefit of olaparib maintenance therapy for patients with BRCA-mutated platinum-sensitive relapsed ovarian cancer. J Med Econ. 2015;18(7):516–24. https://doi.org/10.3111/13696998.2015.1024682.
Swedish Association of Local Authorities and Regions. Introduction and follow-up protocol for the national managed introduction of olaparib [Olaparib (Lynparza) som underhållsbehandling vid platinumkänslig recidiverande ovarialcancer-Införande/uppföljningsprotokoll för nationellt ordnat införande av läkemedel (pilotförsök)]. 2015.
Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–73.
Wettermark B, Hammar N, MichaelFored C, Leimanis A, Olausson PO, Bergman U, et al. The new Swedish prescribed drug register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–35.
Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register–a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol. 2016;119(5):464–9.
Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer register–a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33.
Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. https://doi.org/10.1186/1471-2458-11-450.
Regionala cancercentrum. Ovarian Cancer: National Treatment Guidelines [Äggstockscancer: Nationellt vårdprogram]. https://cancercentrum.se/samverkan/cancerdiagnoser/gynekologi/aggstock/vardprogram/. 2015.
Berger ML, Curtis MD, Smith G, Harnett J, Abernethy AP. Opportunities and challenges in leveraging electronic health record data in oncology. Future Oncol. 2016;12(10):1261–74. https://doi.org/10.2217/fon-2015-0043.
Bartlett C, Mardekian J, Cotter M, Huang X, Zhang Z, Parrinello C, et al. Concordance of real world progression free survival (PFS) on endocrine therapy as first line treatment for metastatic breast cancer using electronic health record with proper quality control versus conventional PFS from a phase 3 trial. Cancer Res. 2018;78(4 Supplement):P3-17-03. https://doi.org/10.1158/1538-7445.sabcs17-p3-17-03.
Cars T. Real-Time Monitoring of Healthcare Interventions in Routine Care: Effectiveness and Safety of Newly Introduced Medicines. Uppsala University. 2016.
Cars T, Lindhagen L, Malmstrom RE, Neovius M, Schwieler J, Wettermark B, et al. Effectiveness of drugs in routine care: a model for sequential monitoring of new medicines using Dronedarone as example. Clin Pharmacol Ther. 2018;103(3):493–501. https://doi.org/10.1002/cpt.751.
Cars T, Wettermark B, Lofberg R, Eriksson I, Sundstrom J, Lordal M. Healthcare utilisation and drug treatment in a large cohort of patients with inflammatory bowel disease. J Crohn’s Colitis. 2016;10(5):556–65. https://doi.org/10.1093/ecco-jcc/jjv243.
Cars T, Wettermark B, Malmstrom RE, Ekeving G, Vikstrom B, Bergman U, et al. Extraction of electronic health record data in a hospital setting: comparison of automatic and semi-automatic methods using anti-TNF therapy as model. Basic Clin Pharmacol Toxicol. 2013;112(6):392–400. https://doi.org/10.1111/bcpt.12055.
Acknowledgements
The authors wish to thank Kristina Aggefors for facilitating the conduct of this project; Maria Juhasz Haverinen for help with the acquisition of drug sales data; Håkan Holmberg and Caroline Gredenborn for advice on the Swedish national registers; and Mia von Euler, Anders Viberg, Freddi Lewin, and Jan Liliemark for reading the manuscript and for helpful discussions.
Author information
Authors and Affiliations
Contributions
IE and KB designed the study. IE managed and analyzed the data. IE and KB interpreted the data. IE wrote the manuscript, and BW and KB revised it critically for important intellectual content. All authors approved the version to be submitted for publication. IE had full access to all data and is responsible for the integrity and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Funding
This study was funded by the Swedish Association of Local Authorities and Regions. The funder had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit the manuscript for publication.
Conflict of Interest
IE, BW, and KB declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Electronic supplementary material
ESM 1
(PDF 27.9 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eriksson, I., Wettermark, B. & Bergfeldt, K. Real-World Use and Outcomes of Olaparib: a Population-Based Cohort Study. Targ Oncol 13, 725–733 (2018). https://doi.org/10.1007/s11523-018-0604-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-018-0604-z