Abstract
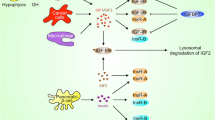
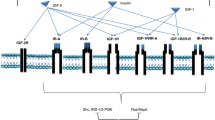
It is believed that the insulin-like growth factor receptor type 1 (IGF-1R) signaling pathway plays a pivotal role in cancer growth, progression, and resistance to anticancer therapies. Strategies are being developed to block IGF-1R as an anticancer treatment. We reviewed several potential strategies for disrupting the IGF axis. We also reviewed the effects of two drugs that target the IGF-1R: monoclonal antibodies and tyrosine kinase inhibitors. Preliminary results of studies involving these agents provided a foundation for ongoing clinical trials, whose results in the near future will help us understand how to incorporate anti IGF-1R strategies into the current anticancer armamentarium.


Similar content being viewed by others
References
Pollak MN, Schernhammer ES, Hankinson SE (2004) Insulin-like growth factors and neoplasia. Nat Rev Cancer 4:505–418
Hankinson SE, Willett WC, Colditz GA et al (1998) Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 351(9113):1393–1396
Roddam AW, Allen NE, Appleby P et al (2008) Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med 149(7):461–471
Ma J, Pollak MN, Giovannucci E et al (1999) Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst 91(7):620–625
Ouban A, Muraca P, Yeatman T et al (2003) Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol 34(8):803–908
Camirand A, Zakikhani M, Young F et al (2005) Inhibition of insulin-like growth factor-1 receptor signaling enhances growth-inhibitory and proapoptotic effects of gefitinib (Iressa) in human breast cancer cells. Breast Cancer Res 7(4):570–579
Jones HE, Goddard L, Gee JM et al (2004) Insulin-like growth factor-I receptor signaling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 11(4):793–814
Chakravarti A, Loeffler JS, Dyson NJ (2002) Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res 62(1):200–207
Liu B, Fang M, Lu Y et al (2001) Fibroblast growth factor and insulin-like growth factor differentially modulate the apoptosis and G1 arrest induced by anti-epidermal growth factor receptor monoclonal antibody. Oncogene 20(15):1913–1922
Sakamoto M, Kondo A, Kawasaki K et al (2001) Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cDNA microarray. Hum Cell 14(4):305–315
Shimizu C, Hasegawa T, Tani Y et al (2004) Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Hum Pathol 35(12):1537–1542
Abe S, Funato T, Takahashi S et al (2006) Increased expression of insulin-like growth factor i is associated with Ara-C resistance in leukemia. Tohoku J Exp Med 209(3):217–228
Cappuzzo F, Toschi L, Tallini G, Ceresoli GL, Domenichini I, Bartolini S et al (2006) Insulin-like growth factor receptor 1 (IGFR-1) is significantly associated with longer survival in non-small-cell lung cancer patients treated with gefitinib. Ann Oncol 17(7):1120–1127
Friedrich N, Haring R, Nauck M et al (2009) Mortality and serum insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. J Clin Endocrinol Metab 94(5):1732–1739
Rowlands MA, Gunnell D, Harris R et al (2009) Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer 124(10):2416–2429
Papadimitrakopoulou VA, Brown EN, Liu DD et al (2006) The prognostic role of loss of insulin-like growth factor-binding protein-3 expression in head and neck carcinogenesis. Cancer Lett 239(1):136–143
Chang YS, Wang L, Suh YA, Mao L, Karpen SJ, Khuri FR et al (2004) Mechanisms underlying lack of insulin-like growth factor-binding protein-3 expression in non-small-cell lung cancer. Oncogene 23(39):6569–6580
Baglietto L, English DR, Hopper JL et al (2007) Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 16(4):763–768
Nedic O, Malenkovic V, Dukanovic B et al (2008) Association of elevated IGFBP-1 with increased IGF-II concentration in patients with carcinoma of the liver. Int J Biol Markers 23(4):225–230
Renehan AG, Jones J, Potten CS et al (2000) Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer 83(10):1344–1350
Rikhof B, van Doorn J, Suurmeijer AJ et al (2009) Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol 20(9):1582–1588
Lin Y, Jiang T, Zhou K et al (2009) Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neuro Oncol. doi:10.1215/15228517-2008-114
Letsch M, Schally AV, Busto R et al (2003) Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and -independent prostate cancers. Proc Natl Acad Sci U S A 100(3):1250–1255
Thorner MO, Strasburger CJ, Wu Z et al (1999) Growth hormone (GH) receptor blockade with a PEG-modified GH (B2036-PEG) lowers serum insulin-like growth factor-I but does not acutely stimulate serum GH. J Clin Endocrinol Metab 84(6):2098–2103
Schreiber I, Buchfelder M, Droste M et al (2007) Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol 156(1):75–82
Yin D, Vreeland F, Schaaf LJ et al (2007) Clinical pharmacodynamic effects of the growth hormone receptor antagonist pegvisomant: implications for cancer therapy. Clin Cancer Res 13(3):1000–1009
Divisova J, Kuiatse I, Lazard Z et al (2006) The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF-7 breast cancer xenograft growth. Breast Cancer Res Treat 98(3):315–327
McCutcheon IE, Flyvbjerg A, Hill H et al (2001) Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg 94(3):487–492
Goya M, Miyamoto S, Nagai K et al (2004) Growth inhibition of human prostate cancer cells in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice by a ligand-specific antibody to human insulin-like growth factors. Cancer Res 64(17):6252–6258
Arteaga CL, Kitten LJ, Coronado EB et al (1989) Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest 84(5):1418–1423
Pollak M (2008) Targeting insulin and insulin-like growth factor signaling in oncology. Curr Opin Pharmacol 8(4):384–392
Munshi S, Hall DL, Kornienko M et al (2003) Structure of apo, unactivated insulin-like growth factor-1 receptor kinase at 1.5 A resolution. Acta Crystallogr D Biol Crystallogr 59(Pt 10):1725–1730
Pollak M (2008) Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab 22(4):625–638
Hofmann F, Garcia-Echeverria C (2005) Blocking the insulin-like growth factor-I receptor as a strategy for targeting cancer. Drug Discov Today 10(15):1041–1047
Cohen BD, Baker DA, Soderstrom C et al (2005) Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751, 871. Clin Cancer Res 11(5):2063–2073
Haluska P, Shaw HM, Batzel GN et al (2007) Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751, 871 in patients with refractory solid tumors. Clin Cancer Res 13(19):5834–5840
Lacy MQ, Alsina M, Fonseca R et al (2008) Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751, 871 in patients with multiple myeloma. J Clin Oncol 26(19):3196–3203
Karp DD, Paz-Ares LG, Blakely LJ et al (2007) Efficacy of the anti-insulin like growth factor I receptor (IGF-IR) antibody CP-751871 in combination with paclitaxel and carboplatin as first-line treatment for advanced non-small cell lung cancer (NSCLC). J Clin Oncol, 2007 ASCO Annual Meeting Proceedings Part I. 25(18S):7506
Karp DD, Pollak MN, Cohen RB, Eisenberg PD, Haluska P, Yin D, Lipton A, Demers L, Leitzel K, Hixon ML, Terstappen LW, Garland L, Paz-Ares LG, Cardenal F, Langer CJ, Gualbert A (2009) Safety, pharmacokinetics and pharmacodynamics of the insulin-like growth factor type 1 receptor inhibitor figitumumab (CP-751, 871) in combination with paclitaxel and carboplatin. J Thorac Oncol. doi:10.1097/JTO.0b013e3181ba2f1d
Study of CP-751,871 in Combination With Sunitinib in Patients With Advanced Solid Tumors. http://clinicaltrials.gov/show/NCT00729833. Accessed 28 Sept 2009
A Safety And Efficacy Study Of The Combination Of Oral PF-00299804 And Intravenous CP-751,871 Given Every 3 Weeks. http://clinicaltrials.gov/show/NCT00728390. Accessed 28 Sept 2009
Study Of CP-751,871 In Combination With Cisplatin And Gemcitabine In Chemotherapy-Naïve Patients With Advanced Non-Small Cell Lung Cancer. http://clinicaltrials.gov/show/NCT00560573. Accessed 28 Sept 2009
Phase 2 Trial Of CP-751,871 And Docetaxel In Advanced Breast Cancer. http://clinicaltrials.gov/ct2/show/NCT00678626. Accessed 28 Sept 2009
Study of CP-751,871 in Combination With Docetaxel and Prednisone in Patients With Hormone Insensitive Prostate Cancer (HRPC). http://clinicaltrials.gov/show/NCT00313781. Accessed 28 Sept 2009
Postel-Vinay S, Okuno S, Schuetze S et al (2008) Safety, pharmacokinetics and preliminary activity of the anti-IGF-IR antibody CP-751,871 in patients with sarcoma. 20th EORTC–NCI–AACR Symposium on Molecular Targets and Cancer Therapeutics. Proceedings Eur J Cancer 6(12) 2008, abstract 388
Study of CP-751,871 In combination with exemestane in postmenopausal women with Hormone Receptor Positive Advanced Breast Cancer. http://clinicaltrials.gov/show/NCT00372996. Accessed 28 Sept 2009
Study Using CP-751,871 In Patients With Stage IV Colorectal Cancer That Has Not Responded To Previous Anti-Cancer Treatments. http://clinicaltrials.gov/show/NCT00560560. Accessed 28 Sept 2009
Trial Of CP-751, 871 And Erlotinib In Refractory Lung Cancer (NSCLC). http://clinicaltrials.gov/show/NCT00673049. Accessed 28 Sept 2009
Carboplatin And Paclitaxel With Or Without CP-751, 871 (An IGF-1R Inhibitor) For Advanced NSCLC Of Squamous, Large Cell And Adenosquamous Carcinoma Histology. http://clinicaltrials.gov/show/NCT00596830. Accessed 28 Sept 2009
Burtrum D, Zhu Z, Lu D et al (2003) A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer Res 63:8912–8921
Rowinsky EK, Youssoufian H, Tonra JR et al (2007) (2007) IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res 13(18 Pt 2):5549s–5555s
Higano CS, Yu EY, Whiting SH et al (2007) A phase I, first in man study of weekly IMC-A12, a fully human insulin like growth factor-I receptor IgG1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol 25(18 S): abstract 3505
Rothenberg ML et al (2007) Phase I dose-escalation study of the anti-IGF-IR recombinant human IgG1 monoclonal antibody (Mab) IMC-A12, administered every other week to patients with advanced solid tumors. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, abstract C84
A Five-Tier, Phase 2 Open-Label Study of IMC-A12 Administered as a Single Agent. http://clinicaltrials.gov/show/NCT00668148. Accessed 28 Sept 2009
Cixutumumab in Treating Patients With Relapsed or Refractory Solid Tumors. http://clinicaltrials.gov/ct/show/NCT00831844. Accessed 28 Sept 2009
A Study of IMC-A12 in Adrenocortical Cancer. http://clinicaltrials.gov/show/NCT00810537. Accessed 28 Sept 2009
Monoclonal Antibody IMC-A12 and Temsirolimus in Treating Patients With Locally Advanced or Metastatic Cancer. http://clinicaltrials.gov/ct/show/NCT00678223. Accessed 28 Sept 2009
Cixutumumab and Temsirolimus in Treating Young Patients With Solid Tumors That Have Recurred or Not Responded to Treatment. http://clinicaltrials.gov/ct/show/NCT00880282. Accessed 28 Sept 2009
IMC-A12 and Temsirolimus in Treating Patients With Locally Recurrent or Metastatic Breast Cancer. http://clinicaltrials.gov/ct/show/NCT00699491. Accessed 28 Sept 2009
Monoclonal Antibody IMC-A12 and Doxorubicin in Treating Patients With Unresectable, Locally Advanced, or Metastatic Soft Tissue Sarcoma. http://clinicaltrials.gov/ct/show/NCT00720174. Accessed 28 Sept 2009
Study of IMC-A12, Alone or in Combination With Cetuximab, in Patients With Recurrent or Metastatic Squamous Cell Carcinoma (MSCC) of the Head and Neck. http://clinicaltrials.gov/show/NCT00617734. Accessed 28 Sept 2009
Capecitabine and Lapatinib With or Without Monoclonal Antibody Therapy in Treating Patients With Previously Treated HER2-Positive Stage IIIB, Stage IIIC, or Stage IV Breast Cancer. http://clinicaltrials.gov/ct/show/NCT00684983. Accessed 28 Sept 2009
Study Using IMC-A12 or IMC-1121B Plus Mitoxantrone and Prednisone in Metastatic Androgen-Independent Prostate Cancer, Following Disease Progression on Docetaxel-Based Chemotherapy. http://clinicaltrials.gov/show/NCT00683475. Accessed 28 Sept 2009
A Study for Safety and Effectiveness of IMCA12 by Itself or Combined With Antiestrogens to Treat Metastatic Breast Cancer in Patients Who Progressed on Antiestrogen Therapy. http://clinicaltrials.gov/show/NCT00728949. Accessed 28 Sept 2009
Mitotane With or Without IMC-A12 in Treating Patients With Recurrent, Metastatic, or Primary Adrenocortical Cancer That Cannot Be Removed By Surgery. http://clinicaltrials.gov/ct/show/NCT00778817. Accessed 28 Sept 2009
Erlotinib With or Without IMC-A12 in Treating Patients With Stage III or Stage IV Non-Small Cell Lung Cancer. http://clinicaltrials.gov/ct/show/NCT00778167. Accessed 28 Sept 2009
Study of Effectiveness of IMC-A12 Antibody Combined With Hormone Therapy Prior to Surgery to Treat Prostate Cancer. http://clinicaltrials.gov/show/NCT00769795. Accessed 28 Sept 2009
Multicenter Study of IMC-A12 in Combination With Depot Octreotide in Patients With Carcinoid or Islet Cell Cancer. http://clinicaltrials.gov/show/NCT00781911. Accessed 28 Sept 2009
A Study of Irinotecan and Cetuximab With or Without IMC-A12 for Treatment of People With Colon or Rectum Cancer Who Got Worse After Their First Treatment With Oxaliplatin and Bevacizumab (FC-4). http://clinicaltrials.gov/show/NCT00845039. Accessed 28 Sept 2009
A Study to Test IMC-A12 With and Without Other Standard Chemotherapies in Patients With Lung Cancer Who Have Not Received Chemotherapy Before. http://clinicaltrials.gov/show/NCT00870870. Accessed 28 Sept 2009
Beltran P, Mitchell P, Hwang D et al (2007) Inhibition of endocrine IGF-1 signaling in normal murine tissues and human tumor xenografts with AMG 479, a fully human anti IGF-1R monoclonal antibody. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, 2007, abstract A67
Calzone F, Chung YA, Cajulis E et al (2008) Domain-specific mechanisms of receptor inhibition by AMG 479, a fully-human IGF1R targeted antibody. 99th AACR Annual Meeting 2008, abstract 3994
Moody G, Mitchell P, Cajulis E et al (2007) AMG 479, a fully human anti IGF-1 receptor monoclonal antibody, is efficacious against Ewing’s sarcoma and osteosarcoma xenografts. AACR-NCI-EORTC International Conference: Molecular Targets and Cancer Therapeutics, 2007, abstract A64
Tolcher AW, Rothenberg ML, Rodon J, et al. A phase I pharmacokinetic and pharmacodynamic study of AMG 479, a fully human monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-1R), in advanced solid tumors. J Clin Oncol2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18 S Suppl; abstr: 3002
Sarantopoulos J, Mita AC, Mulay M et al (2008) A phase IB study of AMG-479, a type 1 insulin-like growth factor receptor (IGF1R) anitbody, in combination with panitumumab (P) or gemcitabine (G). J Clin Oncol 26(15S):3583
A Phase 2 Study of AMG 479 in Relapsed or Refractory Ewing’s Family Tumor and Desmoplastic Small Round Cell Tumors. http://clinicaltrials.gov/show/NCT00563680. Accessed on 28 Sept 2009
Study of AMG 479 as Second Line Therapy in Patients With Recurrent Platinum-Sensitive Ovarian Cancer. http://clinicaltrials.gov/show/NCT00719212. Accessed on 28 Sept 2009
A Study of AMG 655 or AMG 479 in Combination With Gemcitabine for Treatment of Metastatic Pancreatic Cancer. http://clinicaltrials.gov/show/NCT00630552. Accessed on 28 Sept 2009
Panitumumab Combination Study With AMG 102 or AMG 479 in Wild-Type KRAS mCRC. http://clinicaltrials.gov/show/NCT00788957. Accessed on 28 Sept 2009
A Phase 1b/2 Trial of AMG 479 or AMG 102 in Combination With Platinum-Based Chemotherapy as First-Line Treatment for Extensive Stage Small Cell Lung Cancer. http://clinicaltrials.gov/show/NCT00791154. Accessed on 28 Sept 2009
Phase 1b/2 Study of AMG 479 in Combination With Paclitaxel and Carboplatin for 1st Line Treatment of Advanced Squamous Non-Small Cell Lung Cancer. http://clinicaltrials.gov/show/NCT00807612. Accessed on 28 Sept 2009
AMG 655 in Combination With AMG 479 in Advanced, Refractory Solid Tumors. http://clinicaltrials.gov/show/NCT00819169. Accessed on 28 Sept 2009
Amgen Protocol No. 20060362 - A Study of AMG 479 With Exemestane or Fulvestrant in Postmenopausal Women With Hormone Receptor Positive Locally Advanced or Metastatic Breast Cancer. http://clinicaltrials.gov/show/NCT00626106. Accessed on 28 Sept 2009
Phase 2 Safety & Efficacy of FOLFIRI in Combination With AMG 479 or AMG 655 vs FOLFIRI in KRAS-Mutant Metastatic Colorectal Carcinoma. http://clinicaltrials.gov/show/NCT00813605. Accessed on 28 Sept 2009
Goetsch L, Gonzalez A, Leger O et al (2005) Recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer 113:316–328
Atzori F, Tabernero J, Cervantes A et al (2008) A phase I, pharmacokinetic (PK) and pharmacodynamic (PD) study of weekly (qW) MK-0646, an insulin-like growth factor-1 receptor (IGF1R) monoclonal antibody (MAb) in patients (pts) with advanced solid tumors. J Clin Oncol 26(15S): abstract 3519
Hidalgo M, Tirado Gomez M, Lewis N et al (2008) A phase I study of MK-0646, a humanized monoclonal antibody against the insulin-like growth factor receptor type 1 (IGF1R) in advanced solid tumor patients in a q2 wk schedule. J Clin Oncol 26(15S): abstract 3520
A Study of MK0646 in Breast Cancer Patients. http://clinicaltrials.gov/show/NCT00759785. Accessed on 28 Sept 2009
A Combination Study With MK8669 and MK0646 in Patients With Advanced Cancer. http://clinicaltrials.gov/show/NCT00730379. Accessed on 28 Sept 2009
MK-0646 Insulin Growth Factor 1 Receptor Antibody in Stage IIIb or IV Metastatic Non-Squamous Lung Cancer (IMPACT). http://clinicaltrials.gov/show/NCT00799240. Accessed on 28 Sept 2009
Phase I Imaging Study Evaluating MK0646 in Combination With Erlotinib for Patients With Non-Small Cell Lung Cancer. http://clinicaltrials.gov/show/NCT00729742. Accessed on 28 Sept 2009
MK-0646, Etoposide, and Cisplatin in Treating Patients With Extensive-Stage Small Cell Lung Cancer. http://clinicaltrials.gov/ct/show/NCT00869752. Accessed on 28 Sept 2009
Clinical and Translational Study of MK-0646 in Patients With Metastatic Neuroendocrine Tumors (NET). http://clinicaltrials.gov/show/NCT00610129. Accessed on 28 Sept 2009
MK-0646 and Gemcitabine +/- Erlotinib for Patients With Advanced Pancreatic Cancer. http://clinicaltrials.gov/show/NCT00769483. Accessed on 28 Sept 2009
Study of MK0646 in Combination With Cetuximab and Irinotecan in Metastatic Colorectal Cancer. http://clinicaltrials.gov/show/NCT00614393. Accessed on 28 Sept 2009
Rodon J, Patnaik A, Stein M et al (2007) A phase I study of q3W R1507, a human monoclonal antibody IGF-1R antagonist in patients with advanced cancer. J Clin Oncol 25(18S):3590
A Multiple Ascending Dose Study of R1507 in Children and Adolescents With Advanced Solid Tumors. http://clinicaltrials.gov/show/NCT00560144. Accessed on 28 Sept 2009
A Study of R1507 in Patients With Recurrent or Refractory Sarcoma. http://clinicaltrials.gov/show/NCT00642941. Accessed on 28 Sept2009
A Study of the Effect of R1507 in Combination With Tarceva (Erlotinib) on Progression-Free Survival in Patients With Stage IIIb/IV Non-Small Cell Lung Cancer (NSCLC). http://clinicaltrials.gov/show/NCT00760929. Accessed on 28 Sept 2009
A Study of the Effect of R1507 in Combination With Tarceva (Erlotinib) on Progression-Free Survival in Patients With Stage IIIb/IV Non-Small Cell Lung Cancer (NSCLC) Having Received Tarceva Monotherapy. http://clinicaltrials.gov/show/NCT00773383. Accessed on 28 Sept 2009
A Study of R1507 in Combination With Letrozole in Postmenopausal Women With Advanced Breast Cancer. http://clinicaltrials.gov/show/NCT00796107. Accessed on 28 Sept 2009
A Study of R1507 in Combination With Multiple Standard Chemotherapy Treatments in Patients With Advanced Solid Tumors. http://clinicaltrials.gov/show/NCT00811993. Accessed on 28 Sept 2009
Maloney EK, McLaughlin JL, Dagdigian NE et al (2003) An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res 63:5073–5083
Dallas NA, Xia L, Fan F et al (2009) Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res 69(5):1951–1957
Descamps G, Gomez-Bougie P, Venot C et al (2009) A humanised anti-IGF-1R monoclonal antibody (AVE1642) enhances Bortezomib-induced apoptosis in myeloma cells lacking CD45. Br J Cancer 100(2):366–369
Moreau P, Hulin C, Facon T et al (2007) Phase I study of AVE1642 Anti IGF-1R monoclonal antibody in patients with multiple myeloma. ASH Annual Meeting Abstracts. Blood 11:1166
Tolcher AW, Patnaik A, Till E et al (2008) A phase I study of AVE1642, a humanized monoclonal antibody IGF-1R (Insulin like growth factor 1 receptor) antagonist, in patients (pts) with advanced solid tumor (ST). J Clin Oncol 26(15 S): abstract 3582
Dose Finding Study of AVE1642 in Patients With Advanced or Metastatic Liver Carcinoma. http://clinicaltrials.gov/show/NCT00791544. Accessed on 28 Sept 2008
Kolb EA, Gorlick R, Houghton PJ et al (2008) Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer 50(6):1190–1197
Seraj J, Tsai M, Seiberling M et al (2007) Evaluation of safety and pharmacokinetics of a fully human IGF-1 receptor antibody, SCH 717454, in healthy volunteers. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2007, Abstract A72
A Study to Determine the Activity of SCH 717454 in Subjects With Relapsed Osteosarcoma or Ewing’s Sarcoma (Study P04720). http://clinicaltrials.gov/show/NCT00617890. Accessed on 28 September 2008
A Study to Determine the Activity of SCH 717454 in Subjects With Relapsed or Recurrent Colorectal Cancer (Study P04721AM1). http://clinicaltrials.gov/show/NCT00551213. Accessed on 28 September 2008
Hariharan K, Dong J; Demarest S et al (2007) BIIB022, a fully human nonglycosylated γ4P antibody targeting IGF-1R for cancer therapy. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, 2007, abstract B210
Dong J, Tamraz S, Berquist L et al (2008) BIIB022, a human antibody targeting human insulin-like growth factor-1 receptor (IGF-1R), enhances the antitumor activities of Tarceva in non small cell lung carcinoma (NSCLC) and Rapamycin in sarcoma cell lines. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; 2008. Abstr. 4002.
Cortes J, Paquette R, Talpaz M et al (2008) Preliminary Clinical Activity in a Phase I Trial of the BCR-ABL/IGF- 1R/Aurora Kinase Inhibitor XL228 in Patients with Ph++ Leukemias with Either Failure to Multiple TKI Therapies or with T315I Mutation. ASH Annual Meeting Abstracts Blood 112:3232
Smith DC, C. Britten C, Clary DO et al (2009) A phase I study of XL228, a potent IGF1R/AURORA/SRC inhibitor, in patients with solid tumors or hematologic malignancies. J Clin Oncol 27(15S): abstract 3512
Harzstark AL, Ryan C, Diamond M et al (2007) A phase I trial of nordihydroguareacetic acid (NDGA) in patients with non-metastatic prostate cancer and rising PSA. J Clin Oncol 25(18S) abstract 15500
Pharmacokinetic and Efficacy Study of Nordihydroguaiaretic Acid (NDGA) in Non Metastatic Recurrent Prostate Cancer. http://clinicaltrials.gov/show/NCT00678015. Accessed on 28 Sept 2008
Werner H, Woloschak M, Adamo M et al (1989) Developmental regulation of the rat insulin-like growth factor I receptor gene. Proc Natl Acad Sci U S A 86(19):7451–7455
Benyoucef S, Surinya KH, Hadaschik D, Siddle K (2007) Characterization of insulin/IGF hybrid receptors: contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11sequence to ligand binding and receptor activation. Biochem J 403:603–613
Lammers R, Gray A, Schlessinger J, Ullrich A (1989) Differential signalling potential of insulin- and IGF-1-receptor cytoplasmic domains. EMBO J 8:1369–1375
Belfiore A (2007) The role of insulin receptor isoforms and hybrid insulin/IGF-I receptors in human cancer. Curr Pharm Des 13:671–686
Gualberto A, Pollak M (2009) Clinical Development of Inhibitors of the Insulin-like Growth Factor Receptor in Oncology. Curr Drug Targets. Oct 1 epublication
Gualberto A, Karp DD (2009) Development of the monoclonal antibody figitumumab, targeting the insulin-like growth factor-1 receptor, for the treatment of patients with non-small-cell lung cancer. Clin Lung Cancer 10(4):273–280
Olmos D, Molife R, Okuno S, Worden F, Hammer G, Yap T et al (2007) Safety, tolerability and preliminary efficacy of the anti-IGFIR monoclonal antibody CP-751,871 in patients with sarcomas and adrenocortical tumors. Presented at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Meeting, San Francisco, USA, 22–26, October 2007
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351(4):337–345
Thomson S, Buck E, Petti F et al (2005) Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res 65:9455–9462
Pandini G, Wurch T, Akla B et al (2007) Functional responses and in vivo anti-tumour activity of h7C10: a humanised monoclonal antibody with neutralising activity against the insulin-like growth factor-1 (IGF-1) receptor and insulin/IGF-1 hybrid receptors. Eur J Cancer 43:1318–1327
Huang FHW, Hafezi R, Han X et al (2007) Identification of sensitivity markers for BMS-536924, an inhibitor for insulin-like growth factor-1 receptor. ASCO Ann Meet Proc 2007. J Clin Oncol 25:3506
Pollak MNLM, Lipton A, Demers L (2007) Pharmacodynamic properties of the anti-IGF-IR monoclonal antibody CP-751,871 in cancer patients. ASCO Ann Meet Proc 2007. J Clin Oncol 25:3587
Scotlandi K (2006) Targeted therapies in Ewing’s sarcoma. Adv Exp Med Biol 587:13–22
Ji QS, Mulvihill M, Franklin M et al (2007) Properties of small molecule IGF-IR kinase inhibitors in preclinical models. AACR Annual Meeting 2007, Los Angeles (CA): abstract 30.9
Acknowledgements
We thank Carol Pearce for her editorial assistance.
Conflict of interest statement
No funds were received in support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atzori, F., Traina, T.A., Ionta, M.T. et al. Targeting insulin-like growth factor type 1 receptor in cancer therapy. Targ Oncol 4, 255–266 (2009). https://doi.org/10.1007/s11523-009-0123-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-009-0123-z