Abstract
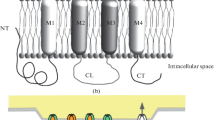
Gap junctions (GJs) are expressed in most cell types of the nervous system, including neuronal stem cells, neurons, astrocytes, oligodendrocytes, cells of the blood brain barrier (endothelial cells and astrocytes) and under inflammatory conditions in microglia/macrophages. GJs connect cells by the docking of two hemichannels, one from each cell with each hemichannel being formed by 6 proteins named connexins (Cx). Unapposed hemichannels (uHC) also can be open on the surface of the cells allowing the release of different intracellular factors to the extracellular space. GJs provide a mechanism of cell-to-cell communication between adjacent cells that enables the direct exchange of intracellular messengers, such as calcium, nucleotides, IP3, and diverse metabolites, as well as electrical signals that ultimately coordinate tissue homeostasis, proliferation, differentiation, metabolism, cell survival and death. Despite their essential functions in physiological conditions, relatively little is known about the role of GJs and uHC in human diseases, especially within the nervous system. The focus of this review is to summarize recent findings related to the role of GJs and uHC in physiologic and pathologic conditions of the central nervous system.




Similar content being viewed by others
References
Abrams CK, Bennett MV, Verselis VK, Bargiello TA (2002) Voltage opens unopposed gap junction hemichannels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proc Natl Acad Sci U S A 99:3980–3984
Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul JY, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS (2008) Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res 86:992–1006
Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, Doms RW, Gonzalez-Scarano F (1999) Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol 73:205–213
Allen K, Fuchs EC, Jaschonek H, Bannerman DM, Monyer H (2011) Gap junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. J Neurosci Off J Soc Neurosci 31:6542–6552
Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL (2002) Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci 22:6458–6470
Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC (2000) Gap junctions: the “kiss of death” and the “kiss of life”. Brain Res Brain Res Rev 32:308–315
Angaut P, Sotelo C (1973) The fine structure of the cerebellar central nuclei in the cat. II. Synaptic organization. Exp Brain Res 16:431–454
Anzini P, Neuberg DH, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R (1997) Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J Neurosci 17:4545–4551
Aronica E, Gorter JA, Jansen GH, Leenstra S, Yankaya B, Troost D (2001) Expression of connexin 43 and connexin 32 gap-junction proteins in epilepsy-associated brain tumors and in the perilesional epileptic cortex. Acta Neuropathol 101:449–459
Ashrafi GH, Pitts JD, Faccini A, McLean P, O’Brien V, Finbow ME, Campo S (2000) Binding of bovine papillomavirus type 4 E8 to ductin (16 K proteolipid), down-regulation of gap junction intercellular communication and full cell transformation are independent events. J Gen Virol 81:689–694
Asklund T, Appelskog IB, Ammerpohl O, Langmoen IA, Dilber MS, Aints A, Ekstrom TJ, Almqvist PM (2003) Gap junction-mediated bystander effect in primary cultures of human malignant gliomas with recombinant expression of the HSVtk gene. Exp Cell Res 284:185–195
Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ (1996) Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS 10:573–585
Ballabh P, Braun A, Nedergaard M (2004) The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16:1–13
Bani-Yaghoub M, Felker JM, Naus CC (1999) Human NT2/D1 cells differentiate into functional astrocytes. Neuroreport 10:3843–3846
Bates DC, Sin WC, Aftab Q, Naus CC (2007) Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia 55:1554–1564
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361:323–331
Benardo LS, Foster RE (1986) Oscillatory behavior in inferior olive neurons: mechanism, modulation, cell aggregates. Brain Res Bull 17:773–784
Bennett MV, Contreras JE, Bukauskas FF, Saez JC (2003) New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617
Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH (1993) Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262:2039–2042
Bittman K, Becker DL, Cicirata F, Parnavelas JG (2002) Connexin expression in homotypic and heterotypic cell coupling in the developing cerebral cortex. J Comp Neurol 443:201–212
Blanc EM, Bruce-Keller AJ, Mattson MP (1998) Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J Neurochem 70:958–970
Blankenship AG, Hamby AM, Firl A, Vyas S, Maxeiner S, Willecke K, Feller MB (2011) The role of neuronal connexins 36 and 45 in shaping spontaneous firing patterns in the developing retina. J Neurosci Off J Soc Neurosci 31:9998–10008
Boisse L, Gill MJ, Power C (2008) HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin 26:799–819, x
Bolanos JP, Medina JM (1996) Induction of nitric oxide synthase inhibits gap junction permeability in cultured rat astrocytes. J Neurochem 66:2091–2099
Bostanci MO, Bagirici F (2006) The effects of octanol on penicillin induced epileptiform activity in rats: an in vivo study. Epilepsy Res 71:188–194
Boucher S, Bennett SA (2003) Differential connexin expression, gap junction intercellular coupling, and hemichannel formation in NT2/D1 human neural progenitors and terminally differentiated hNT neurons. J Neurosci Res 72:393–404
Brand-Schieber E, Werner P, Iacobas DA, Iacobas S, Beelitz M, Lowery SL, Spray DC, Scemes E (2005) Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. J Neurosci Res 80:798–808
Brooks-Kayal AR, Raol YH, Russek SJ (2009) Alteration of epileptogenesis genes. Neurotherapeutics 6:312–318
Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A 100:13644–13649
Burnier L, Fontana P, Angelillo-Scherrer A, Kwak BR (2009) Intercellular communication in atherosclerosis. Physiology (Bethesda) 24:36–44
Butt AM, Ransom BR (1989) Visualization of oligodendrocytes and astrocytes in the intact rat optic nerve by intracellular injection of lucifer yellow and horseradish peroxidase. Glia 2:470–475
Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW (2006) A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol 177:27–39
Carlen PL, Skinner F, Zhang L, Naus C, Kushnir M, Perez Velazquez JL (2000) The role of gap junctions in seizures. Brain Res Brain Res Rev 32:235–241
Chang Q, Balice-Gordon RJ (2000) Gap junctional communication among developing and injured motor neurons. Brain Res Brain Res Rev 32:242–249
Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ (1999) Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal motor neurons. J Neurosci 19:10813–10828
Chang Q, Tang W, Ahmad S, Zhou B, Lin X (2008) Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS One 3:e4088
Cheng A, Tang H, Cai J, Zhu M, Zhang X, Rao M, Mattson MP (2004) Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev Biol 272:203–216
Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL (2005) Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron 46:761–772
Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL (2006) Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol 12:146–152
Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol 66:253–258
Cina C, Maass K, Theis M, Willecke K, Bechberger JF, Naus CC (2009) Involvement of the cytoplasmic C-terminal domain of connexin43 in neuronal migration. J Neurosci 29:2009–2021
Cirenei N, Colombo BM, Mesnil M, Benedetti S, Yamasaki H, Finocchiaro G (1998) In vitro and in vivo effects of retrovirus-mediated transfer of the connexin 43 gene in malignant gliomas: consequences for HSVtk/GCV anticancer gene therapy. Gene Ther 5:1221–1226
Cohen-Salmon M, Regnault B, Cayet N, Caille D, Demuth K, Hardelin JP, Janel N, Meda P, Petit C (2007) Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proc Natl Acad Sci U S A 104:6229–6234
Conant K, Tornatore C, Atwood W, Meyers K, Traub R, Major EO (1994) In vivo and in vitro infection of the astrocyte by HIV-1. Adv Neuroimmunol 4:287–289
Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F (1998) Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci 10:1202–1208
Condorelli DF, Mudo G, Trovato-Salinaro A, Mirone MB, Amato G, Belluardo N (2002) Connexin-30 mRNA is up-regulated in astrocytes and expressed in apoptotic neuronal cells of rat brain following kainate-induced seizures. Mol Cell Neurosci 21:94–113
Condorelli DF, Trovato-Salinaro A, Mudo G, Mirone MB, Belluardo N (2003) Cellular expression of connexins in the rat brain: neuronal localization, effects of kainate-induced seizures and expression in apoptotic neuronal cells. Eur J Neurosci 18:1807–1827
Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC (2002) Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A 99:495–500
Corvalan LA, Araya R, Branes MC, Saez PJ, Kalergis AM, Tobar JA, Theis M, Willecke K, Saez JC (2007) Injury of skeletal muscle and specific cytokines induce the expression of gap junction channels in mouse dendritic cells. J Cell Physiol 211:649–660
Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF (1990) Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol 10:1754–1763
Crow DS, Kurata WE, Lau AF (1992) Phosphorylation of connexin43 in cells containing mutant src oncogenes. Oncogene 7:999–1003
Dahl G, Locovei S (2006) Pannexin: to gap or not to gap, is that a question? IUBMB Life 58:409–419
Dale N (2008) Dynamic ATP signalling and neural development. J Physiol 586:2429–2436
De Pina-Benabou MH, Srinivas M, Spray DC, Scemes E (2001) Calmodulin kinase pathway mediates the K + -induced increase in Gap junctional communication between mouse spinal cord astrocytes. J Neurosci 21:6635–6643
de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U (2000) Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res 86:649–655
de Wit C, Roos F, Bolz SS, Pohl U (2003) Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics 13:169–177
Del Monte U, Statuto M (2004) Drop of connexins: a possible link between aging and cancer? Exp Gerontol 39:273–275
Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MV, Spray DC, Willecke K (1989) Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A 86:10148–10152
Dermietzel R, Hertberg EL, Kessler JA, Spray DC (1991) Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J Neurosci 11:1421–1432
Dermietzel R, Farooq M, Kessler JA, Althaus H, Hertzberg EL, Spray DC (1997) Oligodendrocytes express gap junction proteins connexin32 and connexin45. Glia 20:101–114
Devor A, Yarom Y (2002a) Coherence of subthreshold activity in coupled inferior olivary neurons. Ann N Y Acad Sci 978:508
Devor A, Yarom Y (2002b) Electrotonic coupling in the inferior olivary nucleus revealed by simultaneous double patch recordings. J Neurophysiol 87:3048–3058
Devor A, Yarom Y (2002c) Generation and propagation of subthreshold waves in a network of inferior olivary neurons. J Neurophysiol 87:3059–3069
Dezawa M, Nagano T (1996) Immunohistochemical localization of cell adhesion molecules and cell-cell contact proteins during regeneration of the rat optic nerve induced by sciatic nerve autotransplantation. Anat Rec 246:114–126
Dilber MS, Abedi MR, Christensson B, Bjorkstrand B, Kidder GM, Naus CC, Gahrton G, Smith CI (1997) Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo. Cancer Res 57:1523–1528
Dobrenis K, Chang HY, Pina-Benabou MH, Woodroffe A, Lee SC, Rozental R, Spray DC, Scemes E (2005) Human and mouse microglia express connexin36, and functional gap junctions are formed between rodent microglia and neurons. J Neurosci Res 82:306–315
Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC (2000) Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci 20:RC114
Duffy HS, Sorgen PL, Girvin ME, O’Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC (2002) pH-dependent intramolecular binding and structure involving Cx43 cytoplasmic domains. J Biol Chem 277:36706–36714
Duffy HS, Ashton AW, O’Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC (2004) Regulation of connexin43 protein complexes by intracellular acidification. Circ Res 94:215–222
Dunina-Barkovskaya A (1998) pH dependence of junctional conductance. Membr Cell Biol 11:793–801
Duval N, Gomes D, Calaora V, Calabrese A, Meda P, Bruzzone R (2002) Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J Cell Sci 115:3241–3251
Elble RJ (1996) Central mechanisms of tremor. J Clin Neurophysiol 13:133–144
Elias LA, Kriegstein AR (2008) Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci 31:243–250
Elias LA, Wang DD, Kriegstein AR (2007) Gap junction adhesion is necessary for radial migration in the neocortex. Nature 448:901–907
Elisevich K, Rempel SA, Smith BJ, Edvardsen K (1997) Hippocampal connexin 43 expression in human complex partial seizure disorder. Exp Neurol 145:154–164
Enkvist MO, McCarthy KD (1994) Astroglial gap junction communication is increased by treatment with either glutamate or high K + concentration. J Neurochem 62:489–495
Eugenín EA, Berman JW (2007) Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci 27:12844–12850
Eugenín EA, Eckardt D, Theis M, Willecke K, Bennett MV, Saez JC (2001) Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc Natl Acad Sci U S A 98:4190–4195
Eugenín EA, Branes MC, Berman JW, Saez JC (2003) TNF-alpha plus IFN-gamma induce connexin43 expression and formation of gap junctions between human monocytes/macrophages that enhance physiological responses. J Immunol 170:1320–1328
Eugenín EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood–brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106
Eugenín EA, Gonzalez HE, Sanchez HA, Branes MC, Saez JC (2007) Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro. Cell Immunol 247:103–110
Eugenín EA, Clements JE, Zink MC, Berman JW (2011) Human immunodeficiency virus infection of human astrocytes disrupts blood–brain barrier integrity by a gap junction-dependent mechanism. J Neurosci Off J Soc Neurosci 31:9456–9465
Faccini AM, Cairney M, Ashrafi GH, Finbow ME, Campo MS, Pitts JD (1996) The bovine papillomavirus type 4 E8 protein binds to ductin and causes loss of gap junctional intercellular communication in primary fibroblasts. J Virol 70:9041–9045
Fatemi SH, Folsom TD, Reutiman TJ, Sidwell RW (2008) Viral regulation of aquaporin 4, connexin 43, microcephalin and nucleolin. Schizophr Res 98:163–177
Faustmann PM, Haase CG, Romberg S, Hinkerohe D, Szlachta D, Smikalla D, Krause D, Dermietzel R (2003) Microglia activation influences dye coupling and Cx43 expression of the astrocytic network. Glia 42:101–108
Filson AJ, Azarnia R, Beyer EC, Loewenstein WR, Brugge JS (1990) Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth Differ 1:661–668
Firouzi M, Bierhuizen MF, Kok B, Teunissen BE, Jansen AT, Jongsma HJ, Groenewegen WA (2006a) The human Cx40 promoter polymorphism -44 G–– > A differentially affects transcriptional regulation by Sp1 and GATA4. Biochim Biophys Acta 1759:491–496
Firouzi M, Kok B, Spiering W, Busjahn A, Bezzina CR, Ruijter JM, Koeleman BP, Schipper M, Groenewegen WA, Jongsma HJ, de Leeuw PW (2006b) Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens 24:325–330
Fischer NO, Mbuy GN, Woodruff RI (2001) HSV-2 disrupts gap junctional intercellular communication between mammalian cells in vitro. J Virol Methods 91:157–166
Fonseca CG, Green CR, Nicholson LF (2002) Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res 929:105–116
Frank M, Eiberger B, Janssen-Bienhold U, de Sevilla Muller LP, Tjarks A, Kim JS, Maschke S, Dobrowolski R, Sasse P, Weiler R, Fleischmann BK, Willecke K (2010) Neuronal connexin-36 can functionally replace connexin-45 in mouse retina but not in the developing heart. J Cell Sci 123:3605–3615
Friedman D, Strowbridge BW (2003) Both electrical and chemical synapses mediate fast network oscillations in the olfactory bulb. J Neurophysiol 89:2601–2610
Froger N, Orellana JA, Cohen-Salmon M, Ezan P, Amigou E, Saez JC, Giaume C (2009) Cannabinoids prevent the opposite regulation of astroglial connexin43 hemichannels and gap junction channels induced by pro-inflammatory treatments. J Neurochem 111:1383–1397
Froger N, Orellana JA, Calvo CF, Amigou E, Kozoriz MG, Naus CC, Saez JC, Giaume C (2010) Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol Cell Neurosci 45:37–46
Gajda Z, Szupera Z, Blazso G, Szente M (2005) Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia 46:1581–1591
Galanopoulou AS (2010) Mutations affecting GABAergic signaling in seizures and epilepsy. Pflugers Arch 460:505–523
Galarreta M, Hestrin S (1999) A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature 402:72–75
Gareri P, Condorelli D, Belluardo N, Russo E, Loiacono A, Barresi V, Trovato-Salinaro A, Mirone MB, Ferreri Ibbadu G, De Sarro G (2004) Anticonvulsant effects of carbenoxolone in genetically epilepsy prone rats (GEPRs). Neuropharmacology 47:1205–1216
Gareri P, Condorelli D, Belluardo N, Citraro R, Barresi V, Trovato-Salinaro A, Mudo G, Ibbadu GF, Russo E, De Sarro G (2005) Antiabsence effects of carbenoxolone in two genetic animal models of absence epilepsy (WAG/Rij rats and lh/lh mice). Neuropharmacology 49:551–563
Garg S, Md Syed M, Kielian T (2005) Staphylococcus aureus-derived peptidoglycan induces Cx43 expression and functional gap junction intercellular communication in microglia. J Neurochem 95:475–483
Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, Bennett MV, Abudara V (2010) FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci U S A 107:22659–22664
Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D (1991) Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron 6:133–143
Giaume C, Cordier J, Glowinski J (1992) Endothelins inhibit junctional permeability in cultured mouse astrocytes. Eur J Neurosci 4:877–881
Gibson JR, Beierlein M, Connors BW (1999) Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402:75–79
Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K (2001) Molecular and cellular permeability control at the blood–brain barrier. Brain Res Brain Res Rev 36:258–264
Gonzalez-Nieto D, Gomez-Hernandez JM, Larrosa B, Gutierrez C, Munoz MD, Fasciani I, O’Brien J, Zappala A, Cicirata F, Barrio LC (2008) Regulation of neuronal connexin-36 channels by pH. Proc Natl Acad Sci U S A 105:17169–17174
González-Scarano F, Martín-García J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81
Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF (2003) Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res 1:463–473
Gosejacob D, Dublin P, Bedner P, Huttmann K, Zhang J, Tress O, Willecke K, Pfrieger F, Steinhauser C, Theis M (2011) Role of astroglial connexin30 in hippocampal gap junction coupling. Glia 59:511–519
Granda B, Tabernero A, Sanchez-Abarca LI, Medina JM (1998) The K-ATP channel regulates the effect of Ca2+ on gap junction permeability in cultured astrocytes. FEBS Lett 427:41–45
Grignet-Debrus C, Cool V, Baudson N, Velu T, Calberg-Bacq CM (2000) The role of cellular- and prodrug-associated factors in the bystander effect induced by the Varicella zoster and Herpes simplex viral thymidine kinases in suicide gene therapy. Cancer Gene Ther 7:1456–1468
Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB (1999) ATP released from astrocytes mediates glial calcium waves. J Neurosci 19:520–528
Haefliger JA, Nicod P, Meda P (2004) Contribution of connexins to the function of the vascular wall. Cardiovasc Res 62:345–356
Handel A, Yates A, Pilyugin SS, Antia R (2007) Gap junction-mediated antigen transport in immune responses. Trends Immunol 28:463–466
Harris AL (2001) Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys 34:325–472
Harris AL (2007) Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol
Hartfield EM, Rinaldi F, Glover CP, Wong LF, Caldwell MA, Uney JB (2011) Connexin 36 expression regulates neuronal differentiation from neural progenitor cells. PLoS One 6:e14746
Hauer RN, Groenewegen WA, Firouzi M, Ramanna H, Jongsma HJ (2006) Cx40 polymorphism in human atrial fibrillation. Adv Cardiol 42:284–291
Haughey NJ, Mattson MP (2003) Alzheimer’s amyloid beta-peptide enhances ATP/gap junction-mediated calcium-wave propagation in astrocytes. Neuromol Med 3:173–180
Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H (2001) Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron 31:487–495
Hsiao HJ, Liu PA, Yeh HI, Wang CY (2010) Classical swine fever virus down-regulates endothelial connexin 43 gap junctions. Arch Virol 155:1107–1116
Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL (1998) Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43). Cancer Res 58:5089–5096
Huang RP, Hossain MZ, Sehgal A, Boynton AL (1999) Reduced connexin43 expression in high-grade human brain glioma cells. J Surg Oncol 70:21–24
Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y, Pu PY (2010) The anti-glioma effect of suicide gene therapy using BMSC expressing HSV/TK combined with overexpression of Cx43 in glioma cells. Cancer Gene Ther 17:192–202
Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E (2008) P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295:C752–C760
Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29:7092–7097
Ionasescu VV (1998) X-linked Charcot-Marie-Tooth disease and connexin32. Cell Biol Int 22:807–813
Ionasescu V, Ionasescu R, Searby C (1996) Correlation between connexin 32 gene mutations and clinical phenotype in X-linked dominant Charcot-Marie-Tooth neuropathy. Am J Med Genet 63:486–491
Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y (2011) Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol 193:1257–1274
Iwabuchi S, Kawahara K (2011) Functional significance of the negative-feedback regulation of ATP release via pannexin-1 hemichannels under ischemic stress in astrocytes. Neurochem Int 58:376–384
Jacobson GM, Voss LJ, Melin SM, Mason JP, Cursons RT, Steyn-Ross DA, Steyn-Ross ML, Sleigh JW (2010) Connexin36 knockout mice display increased sensitivity to pentylenetetrazol-induced seizure-like behaviors. Brain Res 1360:198–204
Jahromi SS, Wentlandt K, Piran S, Carlen PL (2002) Anticonvulsant actions of gap junctional blockers in an in vitro seizure model. J Neurophysiol 88:1893–1902
John GR, Scemes E, Suadicani SO, Liu JS, Charles PC, Lee SC, Spray DC, Brosnan CF (1999) IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci U S A 96:11613–11618
Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE (2005) Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience 136:65–86
Kang JQ, Macdonald RL (2009) Making sense of nonsense GABA(A) receptor mutations associated with genetic epilepsies. Trends Mol Med 15:430–438
Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G (2007) Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26:433–443
Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994
Kawasaki A, Hayashi T, Nakachi K, Trosko JE, Sugihara K, Kotake Y, Ohta S (2009) Modulation of connexin 43 in rotenone-induced model of Parkinson’s disease. Neuroscience 160:61–68
Kettenmann H, Orkand RK, Schachner M (1983) Coupling among identified cells in mammalian nervous system cultures. J Neurosci 3:506–516
Kielian T (2008) Glial connexins and gap junctions in CNS inflammation and disease. J Neurochem 106:1000–1016
Kielian T, Esen N (2004) Effects of neuroinflammation on glia-glia gap junctional intercellular communication: a perspective. Neurochem Int 45:429–436
Kleopa KA, Scherer SS (2002) Inherited neuropathies. Neurol Clin 20:679–709
Kleopa KA, Yum SW, Scherer SS (2002) Cellular mechanisms of connexin32 mutations associated with CNS manifestations. J Neurosci Res 68:522–534
Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS (2004) Unique distributions of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia 47:346–357
Knabb MT, Danielsen CA, McShane-Kay K, Mbuy GK, Woodruff RI (2007) Herpes simplex virus-type 2 infectivity and agents that block gap junctional intercellular communication. Virus Res 124:212–219
Koster-Patzlaff C, Hosseini SM, Reuss B (2007) Persistent Borna disease virus infection changes expression and function of astroglial gap junctions in vivo and in vitro. Brain Res 1184:316–332
Koster-Patzlaff C, Hosseini SM, Reuss B (2008) Layer specific changes of astroglial gap junctions in the rat cerebellar cortex by persistent Borna disease virus infection. Brain Res 1219:143–158
Koster-Patzlaff C, Hosseini SM, Reuss B (2009) Loss of connexin36 in rat hippocampus and cerebellar cortex in persistent Borna disease virus infection. J Chem Neuroanat 37:118–127
Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE (2009) A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron 64:133–145
Kreuzberg MM, Deuchars J, Weiss E, Schober A, Sonntag S, Wellershaus K, Draguhn A, Willecke K (2008) Expression of connexin30.2 in interneurons of the central nervous system in the mouse. Mol Cell Neurosci 37:119–134
Kruger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K (2000) Defective vascular development in connexin 45-deficient mice. Development 127:4179–4193
Kubota T, Sato K, Arishima H, Takeuchi H, Kitai R, Nakagawa T (2006) Astroblastoma: immunohistochemical and ultrastructural study of distinctive epithelial and probable tanycytic differentiation. Neuropathology 26:72–81
Kunzelmann P, Blumcke I, Traub O, Dermietzel R, Willecke K (1997) Coexpression of connexin45 and −32 in oligodendrocytes of rat brain. J Neurocytol 26:17–22
Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC (2007) Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res 67:1545–1554
Lee SH, Magge S, Spencer DD, Sontheimer H, Cornell-Bell AH (1995) Human epileptic astrocytes exhibit increased gap junction coupling. Glia 15:195–202
Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L (2005) Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J Comp Neurol 489:1–10
Lemaire I, Falzoni S, Zhang B, Pellegatti P, Di Virgilio F (2011) The P2X7 receptor and Pannexin-1 are both required for the promotion of multinucleated macrophages by the inflammatory cytokine GM-CSF. J Immunol 187:3878–3887
Levite M, Hermelin A (1999) Autoimmunity to the glutamate receptor in mice–a model for Rasmussen’s encephalitis? J Autoimmun 13:73–82
Levite M, Fleidervish IA, Schwarz A, Pelled D, Futerman AH (1999) Autoantibodies to the glutamate receptor kill neurons via activation of the receptor ion channel. J Autoimmun 13:61–72
Li X, Simard JM (2001) Connexin45 gap junction channels in rat cerebral vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 281:H1890–H1898
Li J, Shen H, Naus CC, Zhang L, Carlen PL (2001) Upregulation of gap junction connexin 32 with epileptiform activity in the isolated mouse hippocampus. Neuroscience 105:589–598
Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KG, Yasumura T, Shigemoto R, Rash JE, Nagy JI (2008) Connexin45-containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens-1. J Neurosci Off J Soc Neurosci 28:9769–9789
Liao Y, Day KH, Damon DN, Duling BR (2001) Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A 98:9989–9994
Lim KL, Dawson VL, Dawson TM (2002) The genetics of Parkinson’s disease. Curr Neurol Neurosci Rep 2:439–446
Lin JH, Weigel H, Cotrina ML, Liu S, Bueno E, Hansen AJ, Hansen TW, Goldman S, Nedergaard M (1998) Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci 1:494–500
Lin JH, Takano T, Cotrina ML, Arcuino G, Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, Haubrich S, Willecke K, Goldman SA, Nedergaard M (2002) Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci 22:4302–4311
Locovei S, Wang J, Dahl G (2006) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580:239–244
Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett 581:483–488
Long MA, Deans MR, Paul DL, Connors BW (2002a) Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci Off J Soc Neurosci 22:10898–10905
Long MA, Deans MR, Paul DL, Connors BW (2002b) Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci 22:10898–10905
Long MA, Jutras MJ, Connors BW, Burwell RD (2005) Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci 8:61–66
Lotharius J, Brundin P (2002) Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci 3:932–942
Lynn BD, Marotta CA, Nagy JI (1995) Propagation of intercellular calcium waves in PC12 cells overexpressing a carboxy-terminal fragment of amyloid precursor protein. Neurosci Lett 199:21–24
Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H (2010) Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 58:1104–1117
Magnotti LM, Goodenough DA, Paul DL (2011) Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia 59:26–34
Maier N, Guldenagel M, Sohl G, Siegmund H, Willecke K, Draguhn A (2002) Reduction of high-frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36-deficient mice. J Physiol 541:521–528
Malarkey EB, Parpura V (2008) Mechanisms of glutamate release from astrocytes. Neurochem Int 52:142–154
Mann-Metzer P, Yarom Y (1999) Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci 19:3298–3306
Marconi P, Tamura M, Moriuchi S, Krisky DM, Niranjan A, Goins WF, Cohen JB, Glorioso JC (2000) Connexin 43-enhanced suicide gene therapy using herpesviral vectors. Mol Ther 1:71–81
Marrero H, Orkand RK (1996) Nerve impulses increase glial intercellular permeability. Glia 16:285–289
Martinez AD, Saez JC (1999) Arachidonic acid-induced dye uncoupling in rat cortical astrocytes is mediated by arachidonic acid byproducts. Brain Res 816:411–423
Martínez AD, Eugenin EA, Branes MC, Bennett MV, Saez JC (2002) Identification of second messengers that induce expression of functional gap junctions in microglia cultured from newborn rats. Brain Res 943:191–201
Martini R (2000) Animal models for inherited peripheral neuropathies: chances to find treatment strategies? J Neurosci Res 61:244–250
Matsue H, Yao J, Matsue K, Nagasaka A, Sugiyama H, Aoki R, Kitamura M, Shimada S (2006) Gap junction-mediated intercellular communication between dendritic cells (DCs) is required for effective activation of DCs. J Immunol 176:181–190
Maxeiner S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K (2003) Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience 119:689–700
Mei X, Ezan P, Giaume C, Koulakoff A (2010) Astroglial connexin immunoreactivity is specifically altered at beta-amyloid plaques in beta-amyloid precursor protein/presenilin1 mice. Neuroscience 171:92–105
Meier C, Dermietzel R, Davidson KG, Yasumura T, Rash JE (2004) Connexin32-containing gap junctions in Schwann cells at the internodal zone of partial myelin compaction and in Schmidt-Lanterman incisures. J Neurosci 24:3186–3198
Meldrum BS (1994) The role of glutamate in epilepsy and other CNS disorders. Neurology 44:S14–S23
Meldrum BS, Rogawski MA (2007) Molecular targets for antiepileptic drug development. Neurotherapeutics 4:18–61
Meme W, Ezan P, Venance L, Glowinski J, Giaume C (2004) ATP-induced inhibition of gap junctional communication is enhanced by interleukin-1 beta treatment in cultured astrocytes. Neuroscience 126:95–104
Meme W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C (2006) Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J 20:494–496
Mendoza-Naranjo A, Saez PJ, Johansson CC, Ramirez M, Mandakovic D, Pereda C, Lopez MN, Kiessling R, Saez JC, Salazar-Onfray F (2007) Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J Immunol 178:6949–6957
Mendoza-Naranjo A, Bouma G, Pereda C, Ramirez M, Webb KF, Tittarelli A, Lopez MN, Kalergis AM, Thrasher AJ, Becker DL, Salazar-Onfray F (2011) Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J Immunol
Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2003) Connexins are critical for normal myelination in the CNS. J Neurosci 23:5963–5973
Mesnil M, Piccoli C, Tiraby G, Willecke K, Yamasaki H (1996) Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci U S A 93:1831–1835
Migliore M, Hines ML, Shepherd GM (2005) The role of distal dendritic gap junctions in synchronization of mitral cell axonal output. J Comput Neurosci 18:151–161
Moldrich RX, Chapman AG, De Sarro G, Meldrum BS (2003) Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur J Pharmacol 476:3–16
Moorby C, Patel M (2001) Dual functions for connexins: Cx43 regulates growth independently of gap junction formation. Exp Cell Res 271:238–248
Moortgat KT, Bullock TH, Sejnowski TJ (2000) Gap junction effects on precision and frequency of a model pacemaker network. J Neurophysiol 83:984–997
Morley GE, Taffet SM, Delmar M (1996) Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys J 70:1294–1302
Morley GE, Ek-Vitorin JF, Taffet SM, Delmar M (1997) Structure of connexin43 and its regulation by pHi. J Cardiovasc Electrophysiol 8:939–951
Muller T, Moller T, Neuhaus J, Kettenmann H (1996) Electrical coupling among Bergmann glial cells and its modulation by glutamate receptor activation. Glia 17:274–284
Muller LP, Dedek K, Janssen-Bienhold U, Meyer A, Kreuzberg MM, Lorenz S, Willecke K, Weiler R (2010) Expression and modulation of connexin 30.2, a novel gap junction protein in the mouse retina. Vis Neurosci 27:91–101
Musee J, Mbuy GN, Woodruff RI (2002) Antiviral agents alter ability of HSV-2 to disrupt gap junctional intercellular communication between mammalian cells in vitro. Antiviral Res 56:143–151
Nagy JI, Rash JE (2000) Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev 32:29–44
Nagy JI, Li W, Hertzberg EL, Marotta CA (1996) Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer’s disease. Brain Res 717:173–178
Nagy JI, Ionescu AV, Lynn BD, Rash JE (2003a) Connexin29 and connexin32 at oligodendrocyte and astrocyte gap junctions and in myelin of the mouse central nervous system. J Comp Neurol 464:356–370
Nagy JI, Ionescu AV, Lynn BD, Rash JE (2003b) Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia 44:205–218
Nakase T, Naus CC (2004) Gap junctions and neurological disorders of the central nervous system. Biochim Biophys Acta 1662:149–158
Nakase T, Fushiki S, Naus CC (2003) Astrocytic gap junctions composed of connexin 43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke 34:1987–1993
Nakase T, Sohl G, Theis M, Willecke K, Naus CC (2004) Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol 164:2067–2075
Nakase T, Yoshida Y, Nagata K (2006) Enhanced connexin 43 immunoreactivity in penumbral areas in the human brain following ischemia. Glia 54:369–375
Namba H, Iwadate Y, Kawamura K, Sakiyama S, Tagawa M (2001) Efficacy of the bystander effect in the herpes simplex virus thymidine kinase-mediated gene therapy is influenced by the expression of connexin43 in the target cells. Cancer Gene Ther 8:414–420
Naus CC, Bechberger JF, Paul DL (1991) Gap junction gene expression in human seizure disorder. Exp Neurol 111:198–203
Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J (2005) Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434:83–88
Nelles E, Butzler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stumpel F, Jungermann K, Zielasek J, Toyka KV, Dermietzel R, Willecke K (1996) Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci U S A 93:9565–9570
Nilsen KE, Kelso AR, Cock HR (2006) Antiepileptic effect of gap-junction blockers in a rat model of refractory focal cortical epilepsy. Epilepsia 47:1169–1175
Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K (2003) Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci 23:4549–4559
Ohagen A, Ghosh S, He J, Huang K, Chen Y, Yuan M, Osathanondh R, Gartner S, Shi B, Shaw G, Gabuzda D (1999) Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J Virol 73:897–906
Oliveira R, Christov C, Guillamo JS, de Bouard S, Palfi S, Venance L, Tardy M, Peschanski M (2005) Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol 6:7
Omori Y, Zaidan Dagli ML, Yamakage K, Yamasaki H (2001) Involvement of gap junctions in tumor suppression: analysis of genetically-manipulated mice. Mutat Res 477:191–196
Orellana JA, Hernandez DE, Ezan P, Velarde V, Bennett MV, Giaume C, Saez JC (2010) Hypoxia in high glucose followed by reoxygenation in normal glucose reduces the viability of cortical astrocytes through increased permeability of connexin 43 hemichannels. Glia 58:329–343
Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC (2011a) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem
Orellana JA, Shoji KF, Abudara V, Ezan P, Amigou E, Sáez PJ, Jiang JX, Naus CC, Sáez JC, Giaume C (2011b) Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J Neurosci 31:4962–4977
Orkand RK, Nicholls JG, Kuffler SW (1966) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29:788–806
Orthmann-Murphy JL, Abrams CK, Scherer SS (2008) Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35:101–116
Paemeleire K, Leybaert L (2000) Ionic changes accompanying astrocytic intercellular calcium waves triggered by mechanical cell damaging stimulation. Brain Res 857:235–245
Palmer CA, Geyer JD, Keating JM, Gilliam F, Kuzniecky RI, Morawetz RB, Bebin EM (1999) Rasmussen’s encephalitis with concomitant cortical dysplasia: the role of GluR3. Epilepsia 40:242–247
Palop JJ, Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci 13:812–818
Pang B, Neijssen J, Qiao X, Janssen L, Janssen H, Lippuner C, Neefjes J (2009) Direct antigen presentation and gap junction mediated cross-presentation during apoptosis. J Immunol 183:1083–1090
Parenti R, Campisi A, Vanella A, Cicirata F (2002) Immunocytochemical and RT-PCR analysis of connexin36 in cultures of mammalian glial cells. Arch Ital Biol 140:101–108
Parenti R, Cicirata F, Zappala A, Catania A, La Delia F, Cicirata V, Tress O, Willecke K (2010) Dynamic expression of Cx47 in mouse brain development and in the cuprizone model of myelin plasticity. Glia 58:1594–1609
Parihar MS, Brewer GJ (2010) Amyloid-beta as a modulator of synaptic plasticity. J Alzheimers Dis 22:741–763
Parpura V, Scemes E, Spray DC (2004) Mechanisms of glutamate release from astrocytes: gap junction “hemichannels”, purinergic receptors and exocytotic release. Neurochem Int 45:259–264
Pastor A, Kremer M, Moller T, Kettenmann H, Dermietzel R (1998) Dye coupling between spinal cord oligodendrocytes: differences in coupling efficiency between gray and white matter. Glia 24:108–120
Peinado A, Yuste R, Katz LC (1993a) Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron 10:103–114
Peinado A, Yuste R, Katz LC (1993b) Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex 3:488–498
Perez Velazquez JL, Carlen PL (2000) Gap junctions, synchrony and seizures. Trends Neurosci 23:68–74
Perez Velazquez JL, Carlen PL, Skinner FK (2001) Artificial electrotonic coupling affects neuronal firing patterns depending upon cellular characteristics. Neuroscience 103:841–849
Persidsky Y, Stins M, Way D, Witte MH, Weinand M, Kim KS, Bock P, Gendelman HE, Fiala M (1997) A model for monocyte migration through the blood–brain barrier during HIV-1 encephalitis. J Immunol 158:3499–3510
Petrasch-Parwez E, Habbes HW, Weickert S, Lobbecke-Schumacher M, Striedinger K, Wieczorek S, Dermietzel R, Epplen JT (2004) Fine-structural analysis and connexin expression in the retina of a transgenic model of Huntington’s disease. J Comp Neurol 479:181–197
Planells-Cases R, Jentsch TJ (2009) Chloride channelopathies. Biochim Biophys Acta 1792:173–189
Pu P, Xia Z, Yu S, Huang Q (2004) Altered expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg 107:49–54
Rash JE, Yasumura T, Dudek FE, Nagy JI (2001a) Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci 21:1983–2000
Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI (2001b) Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes 8:315–320
Rash JE, Davidson KG, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson CO, Nagy JI (2005) Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol 34:307–341
Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC (2006) S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A 103:4475–4480
Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, Giaume C (2007) Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27:13781–13792
Retamal MA, Yin S, Altenberg GA, Reuss L (2010) Voltage-dependent facilitation of Cx46 hemichannels. Am J Physiol Cell Physiol 298:C132–C139
Reuss B, Dermietzel R, Unsicker K (1998) Fibroblast growth factor 2 (FGF-2) differentially regulates connexin (cx) 43 expression and function in astroglial cells from distinct brain regions. Glia 22:19–30
Robe PA, Rogister B, Merville MP, Bours V (2000) Growth regulation of astrocytes and C6 cells by TGFbeta1: correlation with gap junctions. Neuroreport 11:2837–2841
Roberts TK, Buckner CM, Berman JW (2010) Leukocyte transmigration across the blood–brain barrier: perspectives on neuroAIDS. Front Biosci 15:478–536
Rose CR, Ransom BR (1997) Regulation of intracellular sodium in cultured rat hippocampal neurones. J Physiol 499(Pt 3):573–587
Rouach N, Tence M, Glowinski J, Giaume C (2002a) Costimulation of N-methyl-D-aspartate and muscarinic neuronal receptors modulates gap junctional communication in striatal astrocytes. Proc Natl Acad Sci U S A 99:1023–1028
Rouach N, Calvo CF, Glowinski J, Giaume C (2002b) Brain macrophages inhibit gap junctional communication and downregulate connexin 43 expression in cultured astrocytes. Eur J Neurosci 15:403–407
Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, Giaume C (2002c) Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell 94:457–475
Rouach N, Koulakoff A, Giaume C (2004a) Neurons set the tone of gap junctional communication in astrocytic networks. Neurochem Int 45:265–272
Rouach N, Calvo CF, Duquennoy H, Glowinski J, Giaume C (2004b) Hydrogen peroxide increases gap junctional communication and induces astrocyte toxicity: regulation by brain macrophages. Glia 45:28–38
Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C (2008) Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322:1551–1555
Rozental R, Morales M, Mehler MF, Urban M, Kremer M, Dermietzel R, Kessler JA, Spray DC (1998) Changes in the properties of gap junctions during neuronal differentiation of hippocampal progenitor cells. J Neurosci 18:1753–1762
Rufer M, Wirth SB, Hofer A, Dermietzel R, Pastor A, Kettenmann H, Unsicker K (1996) Regulation of connexin-43, GFAP, and FGF-2 is not accompanied by changes in astroglial coupling in MPTP-lesioned, FGF-2-treated parkinsonian mice. J Neurosci Res 46:606–617
Sáez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MV (2003a) Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand 179:9–22
Sáez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003b) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Sáez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV (2005) Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711:215–224
Sáez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV (2010) Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316:2377–2389
Samoilova M, Li J, Pelletier MR, Wentlandt K, Adamchik Y, Naus CC, Carlen PL (2003) Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: increased gap junctional function and expression. J Neurochem 86:687–699
Samoilova M, Wentlandt K, Adamchik Y, Velumian AA, Carlen PL (2008) Connexin 43 mimetic peptides inhibit spontaneous epileptiform activity in organotypic hippocampal slice cultures. Exp Neurol 210:762–775
Sánchez HA, Orellana JA, Verselis VK, Saez JC (2009) Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am J Physiol Cell Physiol 297:C665–C678
Sanchez-Alvarez R, Paino T, Herrero-Gonzalez S, Medina JM, Tabernero A (2006) Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia 54:125–134
Santiago MF, Alcami P, Striedinger KM, Spray DC, Scemes E (2010) The carboxyl-terminal domain of connexin43 is a negative modulator of neuronal differentiation. J Biol Chem 285:11836–11845
Sargiannidou I, Ahn M, Enriquez AD, Peinado A, Reynolds R, Abrams C, Scherer SS, Kleopa KA (2008) Human oligodendrocytes express Cx31.3: function and interactions with Cx32 mutants. Neurobiol Dis 30:221–233
Sargiannidou I, Vavlitou N, Aristodemou S, Hadjisavvas A, Kyriacou K, Scherer SS, Kleopa KA (2009) Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects. J Neurosci 29:4736–4749
Schalper KA, Orellana JA, Berthoud VM, Saez JC (2009) Dysfunctions of the diffusional membrane pathways mediated by hemichannels in inherited and acquired human diseases. Curr Vasc Pharmacol 7:486–505
Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ (1998) Connexin32-null mice develop demyelinating peripheral neuropathy. Glia 24:8–20
Schubert T, Maxeiner S, Kruger O, Willecke K, Weiler R (2005) Connexin45 mediates gap junctional coupling of bistratified ganglion cells in the mouse retina. J Comp Neurol 490:29–39
Schweighardt B, Atwood WJ (2001) HIV type 1 infection of human astrocytes is restricted by inefficient viral entry. AIDS Res Hum Retroviruses 17:1133–1142
Seror C et al (2011) Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med
Shinoura N, Chen L, Wani MA, Kim YG, Larson JJ, Warnick RE, Simon M, Menon AG, Bi WL, Stambrook PJ (1996) Protein and messenger RNA expression of connexin43 in astrocytomas: implications in brain tumor gene therapy. J Neurosurg 84:839–845, discussion 846
Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M (2003) Signaling at the gliovascular interface. J Neurosci 23:9254–9262
Siushansian R, Bechberger JF, Cechetto DF, Hachinski VC, Naus CC (2001) Connexin43 null mutation increases infarct size after stroke. J Comp Neurol 440:387–394
Soffer D, Raine CS (1980) Morphologic analysis of axo-glial membrane specializations in the demyelinated central nervous system. Brain Res 186:301–313
Sohl G, Guldenagel M, Beck H, Teubner B, Traub O, Gutierrez R, Heinemann U, Willecke K (2000) Expression of connexin genes in hippocampus of kainate-treated and kindled rats under conditions of experimental epilepsy. Brain Res Mol Brain Res 83:44–51
Sohl G, Maxeiner S, Willecke K (2005) Expression and functions of neuronal gap junctions. Nat Rev Neurosci 6:191–200
Soroceanu L, Manning TJ Jr, Sontheimer H (2001) Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 33:107–117
Sotelo C, Angaut P (1973) The fine structure of the cerebellar central nuclei in the cat. I. Neurons and neuroglial cells. Exp Brain Res 16:410–430
Stout CE, Costantin JL, Naus CC, Charles AC (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277:10482–10488
Sun Y, Tang W, Chang Q, Wang Y, Kong W, Lin X (2009) Connexin30 null and conditional connexin26 null mice display distinct pattern and time course of cellular degeneration in the cochlea. J Comp Neurol 516:569–579
Sutor B, Schmolke C, Teubner B, Schirmer C, Willecke K (2000) Myelination defects and neuronal hyperexcitability in the neocortex of connexin 32-deficient mice. Cereb Cortex 10:684–697
Swayne LA, Sorbara CD, Bennett SA (2010) Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J Biol Chem 285:24977–24986
Tabernero A, Sanchez-Alvarez R, Medina JM (2006) Increased levels of cyclins D1 and D3 after inhibition of gap junctional communication in astrocytes. J Neurochem 96:973–982
Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Doring B, Frisch C, Sohl G, Teubner B, Euwens C, Huston J, Steinhauser C, Messing A, Heinemann U, Willecke K (2003) Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci 23:766–776
Tontsch U, Bauer HC (1991) Glial cells and neurons induce blood–brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res 539:247–253
Tornatore C, Nath A, Amemiya K, Major EO (1991) Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol 65:6094–6100
Tornatore C, Chandra R, Berger JR, Major EO (1994a) HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 44:481–487
Tornatore C, Meyers K, Atwood W, Conant K, Major E (1994b) Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol 68:93–102
Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L (2003) Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol 5:720–726
Tress O, Maglione M, Zlomuzica A, May D, Dicke N, Degen J, Dere E, Kettenmann H, Hartmann D, Willecke K (2011) Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes pelizaeus-merzbacher-like disease in humans. PLoS Genet 7:e1002146
Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK (1999) Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol 113:721–742
Trosko JE, Chang CC (2003) Isolation and characterization of normal adult human epithelial pluripotent stem cells. Oncol Res 13:353–357
Ure JA, Perassolo M (2000) Update on the pathophysiology of the epilepsies. J Neurol Sci 177:1–17
Ure J, Baudry M, Perassolo M (2006) Metabotropic glutamate receptors and epilepsy. J Neurol Sci 247:1–9
Vaney DI (2002) Retinal neurons: cell types and coupled networks. Prog Brain Res 136:239–254
Vaquero J, Oya S, Manrique M, Lozano AP, Bravo G (1978) Cytological alterations in alumina cream experimental epilepsy. Acta Neurochir (Wien) 42:235–243
Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H (2000) Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci U S A 97:10260–10265
Venance L, Glowinski J, Giaume C (2004) Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol 559:215–230
Vis JC, Nicholson LF, Faull RL, Evans WH, Severs NJ, Green CR (1998) Connexin expression in Huntington’s diseased human brain. Cell Biol Int 22:837–847
Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C (2006) The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 26:5438–5447
Weiss JM, Downie SA, Lyman WD, Berman JW (1998) Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood–brain barrier. J Immunol 161:6896–6903
Wen CM, Cheng YH, Huang YF, Wang CS (2008) Isolation and characterization of a neural progenitor cell line from tilapia brain. Comp Biochem Physiol A Mol Integr Physiol 149:167–180
Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA (2008) Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol 294:H622–H632
Yamaguchi DT, Ma D (2003) Mechanism of pH regulation of connexin 43 expression in MC3T3-E1 cells. Biochem Biophys Res Commun 304:736–739
Yamasaki H, Omori Y, Krutovskikh V, Zhu W, Mironov N, Yamakage K, Mesnil M (1999) Connexins in tumour suppression and cancer therapy. Novartis Found Symp 219:241–254, discussion 254–260
Yao LF, Wang ZK, Wang ZG, Sui D, Zhang LM (2009) Expression and function of Cx32 and Cx43 junctions in medically intractable temporal lobe epilepsy in human. Zhonghua Yi Xue Za Zhi 89:3058–3060
Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci Off J Soc Neurosci 23:3588–3596
Zhang W, Couldwell WT, Simard MF, Song H, Lin JH, Nedergaard M (1999) Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res 59:1994–2003
Zhang W, Nwagwu C, Le DM, Yong VW, Song H, Couldwell WT (2003a) Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J Neurosurg 99:1039–1046
Zhang YW, Nakayama K, Morita I (2003b) A novel route for connexin 43 to inhibit cell proliferation: negative regulation of S-phase kinase-associated protein (Skp 2). Cancer Res 63:1623–1630
Zhang YW, Kaneda M, Morita I (2003c) The gap junction-independent tumor-suppressing effect of connexin 43. J Biol Chem 278:44852–44856
Zhang S, Liang R, Zhou F, Huang X, Ding JH, Hu G (2010) Reversal of rotenone-induced dysfunction of astrocytic connexin43 by opening mitochondrial ATP-sensitive potassium channels. Cell Mol Neurobiol
Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A (2001) hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31:85–94
Zlomuzica A, Reichinnek S, Maxeiner S, Both M, May E, Worsdorfer P, Draguhn A, Willecke K, Dere E (2010) Deletion of connexin45 in mouse neurons disrupts one-trial object recognition and alters kainate-induced gamma-oscillations in the hippocampus. Physiol Behav 101:245–253
Acknowledgments
We apologize to the authors and groups whose work we did not cite due to limitations on the numbers of references. This work was supported by the National Institute of Mental Health grants (MH076679 and MH096625 to E.A.E. and MH075679 and MH083497 to J.W.B.) and by the National Institute of Neurological Disorders and Stroke (NS072238 to FFB, and NS55363 to M.V.L.B, who is the Sylvia and Robert S. Olnick Professor of Neuroscience). We thank the National Multiple Sclerosis Society Grant RG-1001-K-11 and CSR was the Wollowick Family Foundation Professor for Multiple Sclerosis Research (to Dr. Cedric Raine). We thank the NIH Centers for AIDS Research Grant (CFAR) AI-051519, Anillo ATC-71 (JCS) and Centro interdisciplinario de Neurociencias P09-022-F (to JCS) and a CFAR pilot project at the Albert Einstein College of Medicine.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 1
Human astrocytes infected with HIVJR-CSF induce apoptosis and alterations in glutamate metabolism through GJ. (A) HIV infection of astrocytes for 21 days induces apoptosis of the surrounding neurons as determined by TUNEL staining. This apoptosis induced by a few infected astrocytes was GJ dependent, because the addition of AGA (32 μM) reduced bystander neuronal apoptosis to control levels. (B) Analyses of glutamate release to the media indicated that HIV-infection increased levels of extracellular glutamate, and that concurrent addition of AGA further enhanced glutamate release, suggesting that extracellular glutamate is not directly responsible for neuronal apoptosis, because when GJ are blocked cells are protected from apoptosis. * Represents significant difference as compared to control conditions (p < 0.005; n = 4). # represents significant differences between HIV infection as compared to HIV infection plus AGA (p < 0.005; n = 3). (PPT 86 kb)
Figure 2
Gap junction channels control release of inflammatory cytokines in rat microglia. Rat microglia cultures treated with LPS (1 μg/ml) and IFN-γ (10 ng/ml), a condition that induces functional GJ coupling in these cells (Eugenin et al. 2001. 2003, 2007), trigger strong secretion of TNF-α, IL1-β and IL-6 (parallel lines bars) as compared to control conditions (white bars). Addition of AGA (32 μM) to the LPS and IFN-γ treated microglia, to block GJ channels, resulted in significant decrease of secretion of TNF-α and IL1-β, but not IL-6 (cross line bars), suggesting that functional GJ are essential to enhance secretion of some inflammatory factors. * Represents significant difference as compared to control conditions (white bars, p < 0.0005; n = 6) and # represents significant differences between LPS and IFN-γ treated microglia as compared to LPS+ IFN-γ and AGA treated microglia (p < 0.007; n = 5). (PPT 103 kb)
Figure 3
HIV-infected PBMC (A) and microglia (B) in HIV encephalitic human tissue express higher levels of Cx43 as compared to uninfected PBMC or uninfected brain tissue, respectively. HIVADA-infected PBMC treated with MCP-1/CCL2 (100 ng/ml) were analyzed by phase contrast (small insert) and confocal microscopy (A) for Cx43 staining. After infection, PBMCs expressed higher levels of Cx43 as compared to the undetectable levels in uninfected cells. Inset shows the phase picture of the cells. (B) Human brain tissue was analyzed by confocal microscopy for Cx43 (B), CD68 (small insert, microglia/macrophages) and phase contrast (small insert). Normal tissue did not show any staining for Cx43 in CD68 positive cells (data not shown). However brain tissue sections obtained from HIV-infected individuals with CNS compromise (encephalitis, HIVE), expressed Cx43 in microglia/macrophages (Micro) in close contact with neurons (Neu). Arrows, indicate areas of potential GJ areas between microglia and neurons. Also lines were drawn to distinguish the cell limit of microglia and neurons. Bar: 70 μm (PBMCs staining) and 180 μm (tissue staining). (PPT 1159 kb)
Rights and permissions
About this article
Cite this article
Eugenin, E.A., Basilio, D., Sáez, J.C. et al. The Role of Gap Junction Channels During Physiologic and Pathologic Conditions of the Human Central Nervous System. J Neuroimmune Pharmacol 7, 499–518 (2012). https://doi.org/10.1007/s11481-012-9352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-012-9352-5