Abstract
Diffuse intrinsic brainstem gliomas (DIBG) account for 1–2 % of adult gliomas. Their biological characteristics are scarcely understood and whether DIBG are biologically different from supratentorial gliomas remains to be established. We analyzed 17 DIBG samples for IDH1 R132H, alpha internexin, p53, and Ki67 expression, and, in a subset with sufficient DNA amount, for IDH1 and histone H3 mutational status, genomic profiling and MGMT promoter methylation status. A series of 738 adult supratentorial gliomas was used for comparison. Median age at diagnosis was 41 years (range 18.9–65.3 years). Median overall survival was 48.7 months (57 months for low-grade vs. 16 months for high-grade gliomas, p < 0.01). IDH1 sequencing revealed two mutations (IDH1 R132G, IDH1 R132C) out of 7 DIBG whereas the R132H IDH1 enzyme was detected in 1/17 DIBG, suggesting that IDH1 mutations are mostly non R132H in DIBG (2/2), in contrast to supratentorial gliomas (31/313; p = 0.01). Mutations in histone genes H3F3A (encoding H3.3) and HIST1H3B (encoding H3.1) were found in 3/8 (37.5 %) of the DIBG (two H3F3A K27M and one HIST1H3B K27M) versus 6/205 (2.9 %) of the supratentorial high-grade gliomas (four H3F3A G34R and two H3F3A K27M) (p = 0.002). The CGH array showed a higher frequency of chromosome arm 1q gain, 9q gain and 11q loss in DIBG compared to the supratentorial high-grade gliomas, which had a less frequent chromosome 7 gain, and a less frequent chromosome 10 loss. No EGFR amplification was found. These data suggest that adult DIBG differ from adult supratentorial gliomas. In particular, histone genes (H3F3A K27M, HIST1H3B K27M) mutations are frequent in adult DIBG whereas IDH1 R132H mutations are rare.
Similar content being viewed by others
Introduction
Brainstem tumors account for 1–2 % of the primary central nervous system tumors in adults and 10 % in children [1]. Brainstem gliomas (BSG) are the most common neoplasm in this location. Because histological confirmation is often lacking, BSG are classified according to location with diffuse intrinsic brainstem gliomas (DIBG) infiltrating the pons (DIPG), more rarely medulla oblongata and midbrain, and being the most frequent type of BSG in adults [2]. The better prognosis of adult versus pediatric DIBG is not fully explained by histological differences [3], and suggests that the two diseases involve distinct pathways.
Several recurrent genetic alterations have been reported in paediatric DIPG such as PDGFRA amplification [4], P13KCA mutation [5], gain of PARP-1 [6] and more recently H3 histone mutations [7]. In contrast, no such data have been published on adult DIBG, because of the rarity of the disease and because biopsy is generally not performed. This observation prompted us to review our database and to analyze a series of adult DIBG for whom pathological samples were available for molecular studies. We compared these data with the features reported in pediatric DIPG and with our large series of supratentorial gliomas.
Patients and methods
DIBG cases were selected retrospectively from our database (2000–2012) according to the following criteria: (i) An MRI aspect of DIBG (pons, medulla oblongata or midbrain), showing diffuse brainstem T2 hyperintensity involving more than 50 % of the brainstem diameter, with or without contrast-enhancement on T1 sequences; supratentorial, multifocal or cerebellar gliomas infiltrating secondarily the brainstem were excluded, (ii) diagnosis of glioma confirmed by histopathological review, excluding pilocytic astrocytomas, (iii) available tumor tissue for immunohistochemistry and/or genomic assessment, (iv) clinical data of follow-up and outcome and (v) signed informed consent for molecular analysis.
A series of 738 adult supratentorial gliomas (185 grade II, 250 grade III and 303 grade IV) with complete follow-up, array-based comparative genomic hybridization (CGH-array) analysis and IDH1 mutational status, was selected from our database and used as a comparative group.
Immunohistochemical detection of IDH1, p53, INA and Ki67 expression
Automated Immunohistochemistry (IHC) was performed on 4 μm thick FFPE sections with an avidin biotin peroxidase complex on Benchmark XT (Ventana Medical Systems Inc, Tucson, AZ, USA) with the Ventana kit including DAB reagent. The cases were screened for GFAP (clone 6F2, 1/500, Dako), Ki67 (clone Mib1, 1/100, Dako), alpha internexin (INA) (clone 2E3MOI, 1/100, Novus.Interchim), p53 (clone M7001, 1/100, Dako), and the monoclonal antibody IDH1 R132H (clone H09, 1/100, Dianova). Ki67 expression was scored in percentage by counting the immunostained nuclei in 400 cells in the most positive area. P53 and INA expressions were scored as positive when more than 10 % of cells were positive, with the presence of at least one cluster for INA [8].
DNA extraction
DNA was extracted from formalin-fixed paraffin-embedded (FFPE) samples (11 cases) or frozen samples (six cases). DNA was extracted from frozen tissue samples using the iPrep ChargeSwitch® gDNA Tissue Kit (Life Technologies). For FFPE samples, DNA was extracted from 10 sections of 20 μm thickness using the iPrep ChargeSwitch® Forensic Kit (Life Technologies). Extracted DNA was quantified using the NanoDrop 8000 spectrophotometer (Thermo Scientific®). Blood DNA was extracted by conventional saline method.
Oligonucleotides-based array comparative genomic hybridization (CGH array)
CGH array was performed using 3 different platforms. CGH NimbleGen 720 K (Roche Diagnostic NimbleGen®) array was performed following manufacturer instructions. Briefly, blood DNA and frozen tissue DNA were fragmented by heating at 95 °C for 30 min. Fragmented blood DNA and tumor DNA (1 μg) were labelled with Cy3 and Cy5 fluorescent dUTP respectively, using Genomic DNA Enzymatic Labeling Kit (Agilent Technology). Microcon YM 30 spin columns (millipore) were used to remove the unincorporated nucleotides and dyes. Hybridizations were performed on a Nimblegen 720 K oligonucleotide array (nine samples). Then, arrays were incubated in a hybridization oven at 42 °C at 20 rpm for 40 h. Hybridized arrays were then washed following the manufacturer’s instructions. Microarray slides were scanned on a Nimblegen MS200 Microarray Scanner at a 2 μm resolution. Feature extraction was done with DEVA Software (Roche Diagnostics NimbleGen®). Extracted data were imported and analysed using Nexus 6.0 (Biodiscovery). Bacterial artificial chromosome array genomic hybridization was performed for frozen samples as previously described [9]. SNP genotyping was performed for frozen samples on Human 610-Quad arrays using the Illumina Technology (Integragen).
Microsatellites analysis or loss of heterozygosity (LOH) detection
LOH on chromosomes 1p (D1S2736, D1S2667, D1S255 and D1S2890), 19q (D19S420), 9p (D9S169), and 10q was detected by microsatellite analysis on blood and tumor DNA, as previously reported [10].
Methylation sensitive high resolution melting: MGMT promoter
Tumor DNA was bisulfilte-converted using the EZ DNA Methylation Gold kit (Zymo Research Corporation) following the manufacturer’s instructions. Briefly, tumor DNA was bisulfite-converted using the following program: 98 °C, 10 min; 64 °C, 2.5 h. PCR amplification of bisulfited DNA was then performed with the LightCycler 480 (Roche Diagnostics Corporation) as previously described [11].
Sanger sequencing for IDH1 132 , IDH2 172 , BRAF 600 and histone H3 mutations
Codon 132 of IDH1, codon 172 of IDH2, codons 27 and 34 of H3F3A and the codon 27 of HIST1H3B were sequenced using the Sanger method after standard PCR amplification, as previously described [7, 12]. The primer used were as follow: IDH1-F TGTGTTGAGATGGACGCCTATTTG, IDH1-R TGCCACCAACGACCAAGTCA; IDH2-F 5-GCCCGGTCTGCCACAAAGTC, IDH2-R 5-TTGGCAGACTCCAGAGCCCA; BRAF-F: TCATAATGCTTGCTCTGATAGGA and BRAF-R: GGCCAAAAATTTAATCAGTGG; H3F3A-F: GTGATCGTGGCAGGAAAAGT, H3F3A-R: CAAGAGAGACTTTGTCCCATTTT, HIST1H3B-F: GTTTTGCCATGGCTCGTACT and HIST1H3B-R: AAGCGAAGATCGGTCTTGAA. The data were analysed using Chrosmas Lite software.
Statistical analysis
Data analysis was performed using GraphPad Prism software version 5.0. Survival time was measured from the date of diagnosis to the date of last follow-up or death. Survival curves were drawn according to the Kaplan–Meier method with 95 % confidence intervals. Differences between curves were assessed using the log-rank test. χ2 and Fisher’s exact test were used to compare qualitative variables. A p < 0.05 was considered statistically significant.
Results
Seventeen patient cases fulfilled the inclusion criteria (Table 1). Collection of tumor samples and clinico-pathological information was undertaken with informed consent and relevant ethical board approval in accordance with the tenets of the Declaration of Helsinki. There were eight women and nine men with a median age at diagnosis of 41 years (range 18.9–65.3 years) and a median Karnofsky performance status (KPS) of 80 (range 50–90). Initial symptoms were cranial nerve dysfunction in 12 patients (70.5 %), balance disturbance in 10 patients (58.8 %), headaches in seven patients (41.0 %), limb motor weakness in five patients (29.4 %) and cerebellar ataxia in four patients (23.0 %).
Tumors were located in the pons (7/17, 41.0 %), medulla oblongata (3/17, 17.6 %), midbrain (2/17, 11.7 %) and more than one brainstem segment in five patients (29.0 %). Nine cases (53 %) showed contrast-enhancement, including 6 cases with ring-like enhancement, suggesting necrosis. Contrast enhancement was significantly linked to a high histological grade, as it was found in 9/11(82 %) of the high grade gliomas vs. 0/6(0 %) of the low grade ones (p = 0.002). Diffuse infiltrative non-enhancing gliomas were associated with longer survival compared to enhancing lesions (57 vs. 13 months, respectively, p < 0.001).
Clinical course
Fifteen patients received treatment, including radiotherapy in nine cases (5 grade II, 2 grade III and 2 grade IV), concurrent radio-chemotherapy in five cases (2 grade III and 3 grade IV) and neoadjuvant chemotherapy followed by radiotherapy in 1 case (grade II). Median progression free survival was 31.8 months (low-grade 38.1 months vs. 7.6 months in high-grade gliomas, p = 0.001) and median overall survival was 48.7 months (57 months for low-grade vs. 16 months for high-grade gliomas, p < 0.01).
Histopathological examination
Definitive diagnosis after biopsy review included 11 high-grade and 6 low-grade gliomas (Table 1). Median Ki67 staining in low-grade gliomas was 1 % (range 1–8 %) versus 20 % (range 15–50 %) in high grade gliomas. Only one tumor (1/17 = 6 %) expressed the IDH1 R132H mutant protein (vs. 283/738 (38.3 %) of supratentorial gliomas, p = 0.005) and none expressed INA.
Main genomic alterations in DIBG
Eleven high grade DIBG samples (5 grade III, 6 grade IV) and 553 supratentorial gliomas samples were available for genomic array. Copy number alterations were compared with our series of supratentorial adult high-grade gliomas and a previously reported series of paediatric DIPG (Supplementary Figure 1; Supplementary Table 1) [13]. We found more frequent 1q gain, 9q gain and 11q loss and less frequent chromosome 7 gain, 10p loss and 10q loss in DIBG than in supratentorial gliomas. Genomic array detected one PDGRalpha amplification out of 11 (9 %) high grade DIBG (vs. 40/553 (7.2 %) supratentorial grade III and IV gliomas, p = NS (non significant)) and no EGFR amplification (vs. 82/395 (20.7 %) in supratentorial grade III and IV gliomas, p = NS). Our data also suggests that 14q and 16q losses are less common in adult DIBG than in pediatric DIBG.
To further identify significant copy number aberrations on smaller regions (i.e. high copy gains (HCG) and homozygous deletions (HD)), we used the Genomic Identification of Significant Targets in Cancer (GISTIC), with a q value threshold of 0.25 and a G-score of 0.3 as absolute value (lower-0.3 is a copy number losses and greater than 0.3 is a copy number gains). The HCG and HD identified by GISTIC and the potential candidate genes are listed in Supplementary Table 2.
IDH1/IDH2 mutation, H3 mutations, BRAF mutations and MGMT promoter methylation
Out of the seven DNA samples available for IDH1/IDH2 sequencing, two mutations, IDH1 R132C (CGT > TGT) and IDH1 R132G (CGT > GGT), were identified [2/7 (28.6 %) vs. 313/738 (42.4 %), p = 0.7 NS] in supratentorial gliomas (see Supplementary Table 3). It was striking to note that the two IDH1 mutated DIBG were non R132H mutations (2/2 compared to 31/313 in supratentorial gliomas, p = 0.01). Interestingly, the three patients identified with an IDH1 mutation (no. 1, 4 and 7) had a longer survival time (73.2, 70.8 and 56 months). No IDH2 mutations were found.
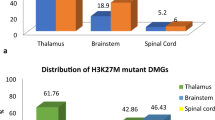
We identified three H3 mutations out of the eight cases with available DNA (37.5 %, see Table 2): H3F3A K27M mutation was detected in one AIII (no. 10, aged 65.3) and one GBM (no. 12, aged 22.6); HIST1H3B K27M mutation in one GBM (no. 15, aged 25.6). No H3F3A G34R mutation was found in DIBG. We also screened a population of 137 supratentorial glioblastomas and 68 grade III gliomas and found H3 mutations in 6 (2.9 %) cases: two grade III gliomas (1 H3F3A K27M and 1 H3F3A G34R) and four glioblastomas (3 H3F3A G34R and 1 H3F3A K27M). The rate of K27 M mutations was significantly higher in DIBG vs supratentorial glioma samples (3/8 vs 2/137; p = 0.001).
We found no BRAF mutation out of 6 samples tested. MGMT promotor methylation was detected in one patient (mutated on IDH1, non mutated on H3) out of six samples tested.
Discussion
We confirm here that adult DIBG have a better outcome than pediatric DIPG. We also show that both the histological grade and the contrast enhancement have a prognostic impact on adult DIBG survival, whereas it appears more controversial in children [3, 14, 15]. It is interesting that 3 out 6 GBM were classified as “GBM-PNET”. This entity, characterised by typical glioblastoma features associated to PNET components (poorly differentiated cells, small round regular nuclei with scant cytoplasm and sharply demarcated hypercellular nodules with neuronal differentiation) is still controversial but may represent a more aggressive type of disease [16]. Indeed our three cases had a poor survival time and the Ki67 percentage was superior to 20 % (Table 1).
Molecular data in adult DIBG are extremely scarce, due to the rarity of the disease and tumor material. In this exploratory study we could retrieve tumor DNA for only a subset of our 17 adult DIBG patients. Due to the multiplicity of testing, these results should be taken with caution. The most robust results are the lower frequency of chromosomes 7 gain in adult DIBG compared to adult supratentorial gliomas (p = 0.004) and the lower frequency of 14q in adult DIBG compared to pediatric DIBG (p = 0.004). The gain of chromosome 7 is extremely frequent in supratentorial astrocytic tumors, and the loss of 14q has been found in two independent series of pediatric DIBG [17, 18].
IDH1/IDH2 mutations are very infrequent in pediatric DIBG [19]. Our data also suggest that IDH1/IDH2 mutations in adult DIBG are mostly IDH1non R132H. This result is in strong opposition to supratentorial gliomas where IDH1 R132H represents nearly 95 % of IDH1 mutations. With slight differences, all the mutant IDH1 neoenzymes lead to the accumulation of 2-d-hydroxyglutarate (2-HG) which is responsible for the CpG island hypermethylated phenotype [20]. Therefore, it is not clear why the R132H mutation is prevalent in supratentorial gliomas [12], but not in DIBG, and in other frequently IDH1 mutated tumors like cartilaginous tumors [21], cholangiocarcinomas where IDH1R132C is predominant [22].
The K27 M mutation of H3 histone has been reported in 75 % (69/92) of pediatric DIBG, and also in pediatric and young adult supratentorial glioblastoma [7, 19, 23, 24]. We show here that the K27 M mutation may be found in adult and even elderly DIBG, but at a lower frequency (3/8 vs. 69/92; p = 0.03) [7, 19] as well as in adult supratentorial grade III and IV gliomas (1.5 % of our cases). The G34R mutation was restricted here to supratentorial gliomas, in accordance with published data for pediatric hemispheric glioblastomas [7, 23, 24], but at a lower frequency (2/137 = 1.5 vs. 6/35 = 17 %; p = 0.001). In addition, G34R is associated with a hypomethylated phenotype, whereas K27 M -mostly reported in brainstem and midline gliomas—is associated with a hypermethylated phenotype, such as IDH mutated tumors [24].
Not surprisingly we found no BRAF 600 mutations. BRAF 600 mutations are frequent in pleiomorphic xanthastrocytomas, gangliogliomas and pilocytic astrocytomas [25], and also reported in diffuse supratentorial but not infratentorial diffuse pediatric gliomas [26].
We identified a high copy number gain and homozygous deletions that contain new potential oncogenes and tumor suppressor genes (listed in Supplementary Table 2): PTPRD (Protein tyrosine phosphatase receptor delta) on 9p23 which acts as a tumor suppressor in neuroblastoma by destabilizing the aurora kinase A oncogene and subsequently N-Myc protein [27], RABGAP1L on 1q25.1, which is rearranged in acute myeloid leukemia [28]. STK19 on 6p21.32, which is mutated in melanoma [29], and CSMD1 on 8p23.2 inactivated in A375 melanoma cells and resulting in Smad pathway activation [30].
In conclusion this first study of histopathological and genomic alterations in adult DIBG suggests that adult DIBG are distinct from both adult supratentorial gliomas and pediatric DIBG. Because of the limited number of cases, the hypothesis raised here must be confirmed by larger systematic studies.
References
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol 14(Suppl 5):v1–v49
Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, Haie-Meder C, Defer GL, Maison P, Mazeron JJ, Cornu P, Delattre JY, Association des Neuro-Oncologues d’Expression Francaise (ANOCEF) (2001) Brainstem gliomas in adults: prognostic factors and classification. Brain 124:2528–2539
Selvapandian S, Rajshekhar V, Chandy MJ (1999) Brainstem glioma: comparative study of clinico-radiological presentation, pathology and outcome in children and adults. Acta Neurochir (Wien) 141:721–726 discussion 726–727
Puget S, Philippe C, Bax DA, Job B, Varlet P, Junier MP, Andreiuolo F, Carvalho D, Reis R, Guerrini-Rousseau L, Roujeau T, Dessen P, Richon C, Lazar V, Le Teuff G, Sainte-Rose C, Geoerger B, Vassal G, Jones C, Grill J (2012) Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS ONE 7:e30313
Grill J, Puget S, Andreiuolo F, Philippe C, MacConaill L, Kieran MW (2012) Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer 58:489–491
Zarghooni M, Bartels U, Lee E, Buczkowicz P, Morrison A, Huang A, Bouffet E, Hawkins C (2010) Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 28:1337–1344
Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Zhang J, Baker SJ (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253
Mokhtari K, Ducray F, Kros JM, Gorlia T, Idbaih A, Taphoorn M, Wesseling P, Hoang-Xuan K, Van den Bent M, Sanson M (2011) Alpha-internexin expression predicts outcome in anaplastic oligodendroglial tumors and may positively impact the efficacy of chemotherapy: European organization for research and treatment of cancer trial 26951. Cancer 117:3014–3026
Idbaih A, Marie Y, Pierron G, Brennetot C, Hoang-Xuan K, Kujas M, Mokhtari K, Sanson M, Lejeune J, Aurias A, Delattre O, Delattre JY (2005) Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol 58:483–487
He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S, Leuraud P, Capelle L, Delattre JY, Poirier J, Hoang-Xuan K (2001) Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol 60:863–871
Quillien V, Lavenu A, Karayan-Tapon L, Carpentier C, Labussiere M, Lesimple T, Chinot O, Wager M, Honnorat J, Saikali S, Fina F, Sanson M, Figarella-Branger D (2012) Comparative assessment of 5 methods (methylation-specific polymerase chain reaction, MethyLight, pyrosequencing, methylation-sensitive high-resolution melting, and immunohistochemistry) to analyze O6-methylguanine-DNA-methyltranferase in a series of 100 glioblastoma patients. Cancer 118:4201–4211
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27:4150–4154
Paugh BS, Qu C, Jones C, Liu Z, Adamowicz-Brice M, Zhang J, Bax DA, Coyle B, Barrow J, Hargrave D, Lowe J, Gajjar A, Zhao W, Broniscer A, Ellison DW, Grundy RG, Baker SJ (2010) Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol 28:3061–3068
Dellaretti M, Reyns N, Touzet G, Dubois F, Gusmao S, Pereira JL, Blond S (2012) Diffuse brainstem glioma: prognostic factors. J Neurosurg 117:810–814
Hargrave D, Chuang N, Bouffet E (2008) Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. J Neurooncol 86:313–319
Perry A, Miller CR, Gujrati M, Scheithauer BW, Zambrano SC, Jost SC, Raghavan R, Qian J, Cochran EJ, Huse JT, Holland EC, Burger PC, Rosenblum MK (2009) Malignant gliomas with primitive neuroectodermal tumor-like components: a clinicopathologic and genetic study of 53 cases. Brain Pathol 19:81–90
Barrow J, Adamowicz-Brice M, Cartmill M, MacArthur D, Lowe J, Robson K, Brundler MA, Walker DA, Coyle B, Grundy R (2011) Homozygous loss of ADAM3A revealed by genome-wide analysis of pediatric high-grade glioma and diffuse intrinsic pontine gliomas. Neuro Oncol 13:212–222
Paugh BS, Broniscer A, Qu C, Miller CP, Zhang J, Tatevossian RG, Olson JM, Geyer JR, Chi SN, da Silva NS, Onar-Thomas A, Baker JN, Gajjar A, Ellison DW, Baker SJ (2011) Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 29:3999–4006
Khuong-Quang DA, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, Bartels U, Albrecht S, Schwartzentruber J, Letourneau L, Bourgey M, Bourque G, Montpetit A, Bourret G, Lepage P, Fleming A, Lichter P, Kool M, von Deimling A, Sturm D, Korshunov A, Faury D, Jones DT, Majewski J, Pfister SM, Jabado N, Hawkins C (2012) K27 M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124:439–447
Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA (2012) IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483:479–483
Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O’Donnell P, Grigoriadis A, Diss T, Eskandarpour M, Presneau N, Hogendoorn PC, Futreal A, Tirabosco R, Flanagan AM (2011) IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol 224:334–343
Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ (2012) Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 17:72–79
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482:226–231
Sturm D, Witt H, Hovestadt V, Khuong-Quang D-A, Jones D, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, Kool M, Zapatka M, Becker N, Zucknick M, Hielscher T, Liu X-Y, Fontebasso AM, Ryzhova M, Albrecht S, Jacob K, Wolter M, Ebinger M, Schuhmann MU, van Meter T, Frühwald M, Hauch H, Pekrun A, Radlwimmer B, Niehues T, von Komorowski G, Dürken M, Kulozik A, Madden J, Donson A, Foreman N, Drissi R, Fouladi M, Scheurlen W, von Deimling A, Monoranu C, Roggendorf W, Herold-Mende C, Unterberg A, Kramm C, Felsberg J, Hartmann C, Wiestler B, Wick W, Milde T, Witt O, Lindroth A, Schwartzentruber J, Faury D, Fleming A, Zakrzewska M, Liberski P, Zakrzewski K, Hauser P, Garami M, Klekner A, Bognar L, Morrissy S, Cavalli F, Taylor M, van Sluis P, Koster J, Versteeg R, Volckmann R, Mikkelsen T, Aldape K, Reifenberger G, Collins VÂP, Majewski J, Korshunov A, Lichter P, Plass C, Jabado N, Pfister S (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A (2011) Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol 121:397–405
Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW (2013) Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602–612
Meehan M, Parthasarathi L, Moran N, Jefferies CA, Foley N, Lazzari E, Murphy D, Ryan J, Ortiz B, Fabius AW, Chan TA, Stallings RL (2012) Protein tyrosine phosphatase receptor delta acts as a neuroblastoma tumor suppressor by destabilizing the aurora kinase A oncogene. Mol Cancer 11:6
Roberti MC, La Starza R, Surace C, Sirleto P, Pinto RM, Pierini V, Crescenzi B, Mecucci C, Angioni A (2009) RABGAP1L gene rearrangement resulting from a der(Y)t(Y;1)(q12;q25) in acute myeloid leukemia arising in a child with Klinefelter syndrome. Virchows Arch 454:311–316
Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DS, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L (2012) A landscape of driver mutations in melanoma. Cell 150:251–263
Tang MR, Wang YX, Guo S, Han SY, Wang D (2012) CSMD1 exhibits antitumor activity in A375 melanoma cells through activation of the Smad pathway. Apoptosis 17:927–937
Acknowledgments
Work supported by the Ligue Nationale contre le Cancer (LNCC) and l’Institut National du cancer (INCa). German Reyes-Botero and Marine Giry have been funded by the ARTC (Association pour la Recherche sur les tumeurs cérébrales). We are indebited to Alexandru Agachi for editing the manuscript.
Disclosures
German Reyes-Botero, Marine Giry, Karima Mokhtari, Marianne Labussière, Ahmed Idbaih, Jean-Yves Delattre, Florence Laigle-Donadey and Marc Sanson have no potential conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reyes-Botero, G., Giry, M., Mokhtari, K. et al. Molecular analysis of diffuse intrinsic brainstem gliomas in adults. J Neurooncol 116, 405–411 (2014). https://doi.org/10.1007/s11060-013-1312-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1312-2