Abstract
Differentiation of posttherapy radiation necrosis from recurrent brain tumor remains a challenging diagnostic problem. The combination of the imaging modalities on the basis of different physiologic mechanisms could improve diagnostic accuracy. The present study assessed the role of 13N-NH3 PET in differentiating recurrent cerebral astrocytoma from radiation necrosis.
Methods
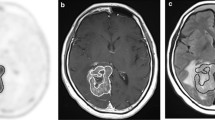
Seven patients, who were previously treated with conventional external-beam radiation therapy after surgical resection for cerebral astrocytomas, and showed the enhancing brain lesions on T1-weighted gadiolinium-enhanced MR studies performed in 6 months or above after the radiotherapies, were examined prospectively with 13N-NH3 and FDG PET. Five lesions with tumor recurrence and two with radiation necrosis were histologically verified by either surgical resection or stereotactic biopsy. One lesion of radiation necrosis was confirmed clinicoradiologically.
Results
In all eight lesions the 13N-NH3 PET scans were concordant with the final diagnosis (100%, 8/8). The lesions with recurrent tumor showed moderately to markedly increased 13N-NH3 uptake (grade = 4–5). The lesions with radiation necrosis showed absent or less 13N-NH3 uptake than surrounding area (grade = 1–2). The FDG PET scans were concordant with the final diagnosis in six of eight lesions (75%, 6/8), and there were one false-negative result and one false-positive result. The diagnostic result of 13N-NH3 PET was discordant with FDG PET in two lesions. One lesion with gliosis and radiation necrosis showed slightly increased FDG uptake (grade = 4), but less 13N-NH3 uptake (grade = 2). The other lesion with anaplastic astrocytoma showed moderately increased 13N-NH3 uptake (grade = 4), but slightly less FDG uptake than surrounding area (grade = 2).
Conclusions
The recurrent astrocytomas showed increased 13N-NH3 uptake, and the radiation necrosis showed absent or less 13N-NH3 uptake, and 13N-NH3 seem superior to 18F-FDG for this purpose, suggesting that 13N-NH3 is a promising tracer for separating radiation necrosis from astrocytoma recurrence. However, the patient population in this study was small. Thus, the further studies are needed to settle this issue.




Similar content being viewed by others
References
Langlebe DD, Segall GM (2000) PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med 41:1861–1867
Janus TJ, Kim EE, Tilbury R et al (1993) Use of [18F]fluorodeoxyglucose positron emission tomography in patients with primary malignant brain tumors. Ann Neurol 33:540–527
Ricci PE, Karis JP, Heiserman JE et al (1998) Differentiating recurrent tumor from radiation necrosis: time for tr-evaluation of positron emission tomography? Am J Neuroradial 9:407–413
Zhang X, Liang C, Chen W et al (2006) PET imaging of cerebral astrocytoma with 13N-ammonia. J Neurooncol 78:145–151
Zhang X, Chen W, Zhou D (2005) 13N-ammonia positron emission tomography in the diagnosis of astrocytomas: preliminary result. J Nucl Med 46(S):429
Kim CK, Alavi JB, Alavi A et al (1991) New grading system of cerebral gliomas using position emission tomography with F-18 fluorodeoxyglucose. J Neurooncol 10:85–91
Phelps ME, Huang SC, Hoffman EJ et al (1981) Cerebral extraction of N-13 ammonia: its dependence on cerebral blood flow and capillary permeability-surface area product. Stroke 12:607–619
Cooper AJL, McDonald JM, Gelbard AS et al (1979) The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem 254:4982–4992
Schelstraete K, Simons M, Deman J et al (1982) Uptake of 13N-ammonia by human tumours as studied by positron emission tomography. Br J Radiol 55:797–804
Sugahara T, Korogi Y, Tomiguchi S et al (2000) Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. Am J Neuroradial 21:901–909
Tsui EY, Chan JH, Leung TW et al (2000) Radionecrosis of the temporal lobe: dynamic susceptibility contrast MRI. Neuroradiology 42:149–152
Schwartz RB, Carvalho PA, Alexander E et al (1991) Radiation necrosis vs high-grade recurrent glioma: differentiation by using dual-isotope SPECT with 201TI and 99mTc-HMPAO. Am J Neuroradiol 12:1187–1192
Carvalho PA, Schwartz RB, Alexander E et al (1992) Detection of recurrent gliomas with quantitative thallium-201/technetium-99m HMPAO single-photon emission computerized tomography. J Neurosurg 77:565–570
Fucci L, Oliver CN, Coon MF et al (1983) Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Pro Natl Acad Sci 80:1521–1525
Pilkington GJ, Lantos PL (1982) The role of glutamine synthetase in the diagnosis of cerebral tumours. Neuropathol Appl Neurobiol 8:227–236
Akimoto J (1993) Immunohistochemical study of glutamine synthetase expression in normal human brain and intracranial tumors. No To Shinkei 45:362–368
McCormick D, McQuaid S, McCusker et al (1990) A study of glutamine synthetase in normal human brain and intracranial tumours. Neuropathol Appl Neurobiol 16:205–211
Spaeth N, Wyss MT, Weber B et al (2004) Uptake of 18F-fluorocholine, 18F-fluoroethyl-l-tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: implications for separation of radiation necrosis from tumor recurrence. J Nucl Med 45:1931–1938
Acknowledgment
This work was partially supported by grant 031652 from the Nature Sciences Foundation of Guangdong Province, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiangsong, Z., Weian, C. Differentiation of recurrent astrocytoma from radiation necrosis: a pilot study with 13N-NH3 PET. J Neurooncol 82, 305–311 (2007). https://doi.org/10.1007/s11060-006-9286-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9286-y