Abstract
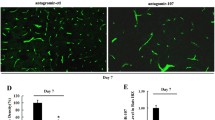
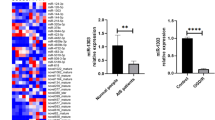
The compensatory angiogenesis that occurs after cerebral ischemia increases blood flow to the injured area and limits extension of the ischemic penumbra. In this way, it improves the local blood supply. Fostering compensatory angiogenesis is an effective treatment for ischemic cerebrovascular disease. However, angiogenesis in the adult organism is a complex, multi-step process, and the mechanisms underlying the regulation of angiogenesis are not well understood. Although Notch signaling reportedly regulates the vascularization process that occurs in ischemic tissues, little is known about the role of Notch signaling in the regulation of ischemia-induced angiogenesis after ischemic stroke. Recent research has indicated that miR-210, a hypoxia-induced microRNA, plays a crucial role in regulating the biological processes that occur in blood vessel endothelial cells under hypoxic conditions. This study was undertaken to investigate the role of miR-210 in regulating angiogenesis in response to brain ischemia injury and the role of the Notch pathway in the body’s response. We found miR-210 to be significantly up-regulated in adult rat ischemic brain cortexes in which the expression of Notch1 signaling molecules was also increased. Hypoxic models of human umbilical vein endothelial cells (HUVE-12) were used to assess changes in miR-210 and Notch1 expression in endothelial cells. Results were consistent with in vivo findings. To determine the molecular mechanisms behind these phenomena, we transfected HUVE-12 cells with miR-210 recombinant lentiviral vectors. We found that miR-210 overexpression caused up-regulation of Notch1 signaling molecules and induced endothelial cells to migrate and form capillary-like structures on Matrigel. These data suggest that miR-210 is involved in the regulation of angiogenesis in response to ischemic injury to the brain. Up-regulation of miR-210 can activate the Notch signaling pathway, which may contribute to angiogenesis after cerebral ischemia.




Similar content being viewed by others
References
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994) Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25:1794–1798
Marti HH, Bernaudin M, Petit E, Bauer C (2000) Neuroprotection and angiogenesis: dual role of erythropoietin in brain ischemia. News Physiol Sci 15:225–229
Hamada Y, Gonda K, Takeda M, Sato A, Watanabe M, Yambe T, Satomi S, Ohuchi N (2011) In vivo imaging of the molecular distribution of the VEGF receptor during angiogenesis in a mouse model of ischemia. Blood 118:e93–e100
Hedhli N, Dobrucki LW, Kalinowski A, Zhuang ZW, Wu X, Russell RR 3rd, Sinusas AJ, Russell KS (2012) Endothelial-derived neuregulin is an important mediator of ischaemia-induced angiogenesis and arteriogenesis. Cardiovasc Res 93:516–524
Gridley T (2007) Vascular biology: vessel guidance. Nature 445:722–723
Hofmann JJ, Luisa Iruela-Arispe M (2007) Notch expression patterns in the retina: an eye on receptor-ligand distribution during angiogenesis. Gene Expr Patterns 7:461–470
Liu R, Trindade A, Sun Z, Kumar R, Weaver FA, Krasnoperov V, Naga K, Duarte A, Gill PS (2012) Inhibition of Notch signaling by Dll4-Fc promotes reperfusion of acutely ischemic tissues. Biochem Biophys Res Commun 418:173–179
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z (2005) Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 37:766–770
Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G (2005) Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 280:9330–9335
Ehebauer M, Hayward P, Arias AM (2006) Notch, a universal arbiter of cell fate decisions. Science 314:1414–1415
Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ (2007) Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA 104:3219–3224
Chan SY, Loscalzo J (2010) MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9:1072–1083
Kelly TJ, Souza AL, Clish CB, Puigserver P (2011) A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol 31:2696–2706
Corn PG (2008) Hypoxic regulation of miR-210: shrinking targets expand HIF-1’s influence. Cancer Biol Ther 7:265–267
Fasanaro P, D’alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F (2008) MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283:15878–15883
Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39:959–966
Jin KL, Mao XO, Nagayama T, Goldsmith PC, Greenberg DA (2000) Induction of vascular endothelial growth factor and hypoxia-inducible factor-1alpha by global ischemia in rat brain. Neuroscience 99:577–585
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 30960396), (Grant No. 81060324) and the National S&T Major Special Project on Major New Drug Innovation (2011ZX09102-010-01).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lou, YL., Guo, F., Liu, F. et al. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem 370, 45–51 (2012). https://doi.org/10.1007/s11010-012-1396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1396-6