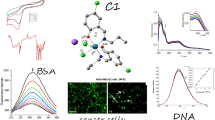
Summary
Rhenium (I)-diselenother (Re-diselenoether) is a water soluble metal-based compound, combining one atom of rhenium and two atoms of selenium. This compound has been reported to exhibit marked activities against several solid tumor cell lines. We now disclose an improved synthesis of this complex. The Re-diselenoether showed a potent inhibitory effect on MDA-MB231 cell division in vitro, which lasted when the complex was no longer present in the culture. Re-diselenoether induced a remarkable reduction of the volume of the primitive breast tumors and of the pulmonary metastases without clinical signs of toxicity, in mice-bearing a MDA-MB231 Luc+ tumor, orthotopically transplanted, after a daily oral administration at the dose of 10 mg/kg/d. Interestingly, an antagonism was observed when cisplatin was administered as a single i.p. injection 1 week after the end of the Re-diselenoether administration. In an effort to gain insight of the mechanisms of action of Re-diselenoether complex, interaction with 9-methylguanine as a nucleic acid base model was studied. We have shown that Re-diselenoether gave both mono- and bis-guanine Re adducts, the species assumed to be responsible for the DNA intrastrand lesions.
Similar content being viewed by others
Introduction
Metal-based drugs have received increasing attention in recent years. The use of metals is indeed very attractive, as they offer unique spectrum of reactivity through ligand exchange and redox processes that is not available in the more common organic-based drugs. The discovery of the anticancer properties of cisplatin during the 1960s spurred the quest for alternative anticancer drugs with less side-effects. Beside platinum analogues including platinum (II) and (IV) derivatives, other metals have been recently explored, such as gallium, ruthenium, iron, gold, titanium or palladium [1]. In this context, rhenium-based drugs appeared as promising candidates for clinical development. Over the past years a growing number of studies have revealed the potential of Re organometallic complexes as anti-cancer agents. A recent review has been published with a particular emphasis on the cellular uptake and the localization of the currently known Re organometallic complexes as well as their potential mechanism of action [2]. Among Re organometallic complexes, several Re carbonyl complexes have been found to display cytotoxicity against breast cancer cell lines. For example, a Re(tricarbonyl)pentylcarbonato compound able to fight triple node negative human breast cancer cell lines has been described [3]. Nevertheless, despite the design of very efficient potent anti-cancer agents, very few in vivo studies have been conducted on cold Re organometallic complexes. On the other hand, it is noteworthy that some Se-based drugs have demonstrated a selective cytotoxicity against cancerous cells [4–6]. The tumor-specific cytotoxic effects of Se, with special emphasis on cascades of cellular events induced by pharmacologically active Se compounds have been recently reviewed [7]. It appears that certain redox-activated Se compounds induce complex cascades of pro-death signaling at pharmacological concentrations with superior tumor specificity, and that the target molecules are often implicated in drug resistance. With the aim to combine the antiproliferative properties of Re with the unique apoptotic modulator properties of Se we have recently designed the rhenium(I)-diselenoether complex 1 in which a central Re atom is coordinated with two Se atoms (Fig. 1). Complex 1 was shown to exhibit remarkable cytotoxicity against MCF-7 breast cancer cell lines [8]. The uptake and efflux of Re in malignant cells exposed to complex 1 have been reported, together with evidence of the incorporation of Re into the nucleus. Furthermore, tissue distribution of Re and Se after oral administration of 1 to mice have been reported [9].
The purpose of the present paper was to report the activity of Re-diselenother complex 1 (Fig. 1) in experimental models of human breast tumor toward highly metastatic MDA-MB231 cancer cells in culture, and in MDA-MB231 Luc+ tumors transplanted in mice. The interaction of 1 with 9-methylguanine is also described, providing evidence that interaction of 1 with DNA might be involved in the mechanism of action of 1 at the molecular level.
Material and methods
Chemical protocols
Although the published synthesis of complex 1 has proved rather efficient, the elaboration of a key intermediate (compound 4 in scheme 1) was somewhat problematic, since suffering from a vexing lack of reproducibility. For that reason, we have recently developed an alternative approach to key-compound 4, which has proved perfectly reproducible. This new protocol involved the alkylation of the disodium salt of propane-diselenocyanate 2 with bromoacetic acid methyl ester, giving diester 3, which was next saponified with lithium hydroxide into diacid 4. Complexation of ReCl(CO)3 by diselenoether 4, followed by sodium bicarbonate treatment provided complex 1, as previously reported [8]. Likewise, to study the possible interactions of the complex with DNA bases without competitive attack of the carboxylate appendages on the Re atom, the corresponding dimethyl ester complex 5 was prepared by condensation of diselenoester 3 with ReCl(CO)3 in 68 % yield.
Synthesis of 1 with optimized approach to key-intermediate 4. Reagents and conditions, i: BrCH2CO2Me, NaBH4, EtOH, 16 h, 20 °C (82 %); ii: LiOH.H2O, THF, MeOH, 16 h, 20 °C (85 %); iii: ReCl(CO)5, THF, reflux, 16 h (72 %); iv: 2.0 equiv. NaHCO3, MeOH,H2O, 0 °C (90 %); v: ReCl(CO)5, THF, reflux, 16 h (68 %)
Alternative synthesis of key intermediate
Preparation of (3-carboxymethylselanyl-propylselanyl)-acetic acid dimethyl ester (compound 3): To a solution of 1,3-bis-selenocyanato-propane 2 (1.0 g, 3.96 mmol) in absolute ethanol (20 mL) was added bromoacetic acid methyl ester (1.23 g, 8.0 mmol). The mixture was stirred under nitrogen until complete dissolution. Sodium borohydride (303 mg, 8.0 mmol) was then added in one portion. The reaction mixture was stirred at room temperature for 16 h. The white precipitate was filtered of on a sintered glass funnel and the paint yellow filtrate was concentrated under reduced pressure to leave 3 as a pale yellow oil (1.12 g, 82 %); 1H NMR (CDCl3): δ 3.72 (s, 6H, OCH 3), 3.17 (s, 4H, CH 2CO2Me), 2.82 (t, J = 7.2 Hz, 4H, SeCH 2CH2CH 2Se), 2.0 (quint, J = 7.2 Hz, 2H, SeCH2CH 2CH2Se).
Preparation of (3-carboxymethylselanyl-propylselanyl)-acetic acid (compound 4)
To a solution of compound 3 (692 mg, 2.0 mmol) in THF (3 mL) and methanol (1 mL) was added a solution of LiOH.H2O (336 mg, 8.0 mmol) in 2 mL of water. The reaction mixture was stirred at room temperature for 16 h and the solvents were removed under reduced pressure. 3 N HCl was added until pH = 1, and the mixture was extracted with ethyl acetate (3 × 15 mL). The combined organic layers were dried over MgSO4 and concentrated under reduced pressure. The oily material was taken into a small amount of CH2Cl2 and precipitate with petroleum ether. The solid was filtered, washed with diethyl ether, and dried under vacuum to give 540 mg (85 % yield) of compound 4, which was unequivocally identified by comparison with an authentic sample.
Synthesis of dimethyl ester complex 5
Preparation of rhenate (tricarbonylchloro[2, 2′-[1, 3-propanediylbis(carbomethoxymethylseleno-)]]) (5): A mixture of diselenoester 3 (216 mg, 0.62 mmol) and ReCl(CO)3 in THF (20 mL) was heated at 60 °C for 16 h. The mixture was cooled to room temperature and concentrated under reduced pressure to leave crude 5. Purification by chromatography over silica gel gave 5 as a colorless oil (278 mg, 68 %); 1H NMR (C6D6): The presence of stereoisomers induced splitting of most signals δ 3.61 (dd, J = 14.1, 4.5 Hz, 1H), 3.42 (dd, J = 14.1, 6.9 Hz, 1H), 3.35–3.24 (m, 6H), 3.16–3.06 (m, 0.5H), 2.99 (d, J = 13.8 Hz, 1H), 2.86 (m, 0.5H), 2.55–2.28 (m, 2 H), 1.5–1.2 (m, 4H).
Interaction of rhenium diseleno-ester 5 with 9-methylguanine
To a solution of complex 5 (139 mg, 0.21 mmol) in methanol (4 mL) was added dropwise a solution of AgBF4 (42 mg, 0.21 mmol) in methanol (1 mL). A sticky precipitate formed immediately. The reaction mixture was stirred at 15 °C for 16 h and filtered. The filtrate was added to a solution of 9-methylguanine (35 mg, 0.21 mmol) in 1:1 water: methanol mixture (24 mL). The reaction mixture was stirred for 3 d at 15 °C and concentrated under reduced pressure (1 mm Hg) at 20 °C. The obtained solid was washed with methylene chloride to remove any trace of free ligand and dried. Mass spectra analysis ESI (+) showed four components: m/z = 782.0 [C18H23N5O8ReSe2]+; 616.9 [C12H16O7ReSe2]+; 601.1 [C15H14N10O5Re]+; 435.9 [C9H7N5O4Re]+ . Ions m/z = 782.0 and m/z = 616.9 showed the characteristic isotopic pattern of the ReSe2 fragment, whereas ions m/z = 601.1 and m/z = 435.9 ions displayed a more simple profile corresponding to a rhenium complex devoid of the diselenoether ligand.
Morphological and inhibitory effects on MDA-MB231 breast cancer cells
Cell lines
MDA-MB-231 (Passage No. 13) breast cancer cell lines were kindly provided by Dr. S. Fraser and Pr. M. Djamgoz at Imperial College, London. Cells were grown as adherent monolayers in Dulbecco’s Modified Eagle Medium (Sigma), supplemented with 5 % Fetal Bovine Serum and phenol red. Cultures were maintained at 37 °C with a humidified atmosphere containing 5 % CO2 and were passaged using 0.25 % trypsin in DPBS (PAA) when they reached 80 % confluency. Cell number was established via haemocytometer count after dead cell exclusion using trypan blue.
In vitro toxicity
MDA-MB231 cells were seeded on 48-well plates at 5 × 104 cells/well, allowed forming an adherent monolayer overnight and then exposed to the indicated concentrations of Re-diselenoether complex for 48 h. Cells were then washed and incubated with Re-diselenoether-free medium for a further 48 h. The effects of Re on cell viability was determined at the indicated time using haemocytometer count of live cells under light microscopy and via flow cytometry. Proliferation. Prior to Re treatment, MDA-MB231 cells at the concentration of 1 × 106 /mL in PBS were labeled with 0.5 M of violet dye (CellTrace violet Invitrogen) for 20 min at 37 °C. The intensity of fluorescence of the violet dye was acquired on an LSRFortessa flow cytometer (BD) and analyzed with FlowJo version 9.3.1 (TreeStar, Ashland, OR, USA). Dose responses over time were analysed using GraphPad Prism software version 5.03 (GraphPad Software Inc.).
Animal study design: oral administration of Re-diselenoether complex
This study was performed in Cellvax laboratory. In this study, one of the objectives was to look for a synergism between cisplatin and Re (I)-diselenoether [10]. Hormone-independant breast cancer MDA-MB231 cells (origin: ATCC#HTB-26™), transfected with the luciferase gene (Luc+) were orthotopically implanted into the mammary gland (fat pad) in athymic nu/nu nude mice (Charles River, France). With a cell viability of about 97 %, 1.0 × 106 cells per mouse were injected in a volume of 50 μl/mouse. The animals were 5 to 6-week-old female, of about 20 g each, and specific and opportunistic pathogen-free. They were acclimatized for at least 7 days before the initiation of the designed study. A total of 30 mice were used for this study. Animals were housed in individual polyethylene cages, in a climate and light-controlled environment. All animals were kept under environmentally controlled housing conditions: lights on between 7:00 AM and 7:00 PM; temperature inside of the animal facility strictly maintained at 21 + 1 °C; relative humidity of 70 % throughout the entire study period, and maintained in accordance with Cellvax approved standard operation procedures (SOP) and with local Ethical Committee approval. Animals were fed with commercially available rodent food (Safe, Les Tremblats, Augy, France). Water (sterilized water) was available ad libitum.
Animals were numbered and given a unique animal identification ear notch mark. Ethical manager . A Ph.D. and Veterinary Doctor at Cellvax company assumed the function of ‘Ethical Manager’ within this project. Experimental groups : Three groups of 10 mice each for a total of 30 mice were treated. Measurable mammary tumors were observed in 18 mice at day 9 after the inoculation of the tumor cells, while no mammary tumors were observed in 12 mice. Groups were then homogeneized to have 6 mice with a measurable tumor in each group. Group 1: Cisplatin (CDDP) group: mice were treated with CDDP as a single intraperitoneal (IP) injection at a dose of 6 mg/kg on day 41 after the inoculation of the tumor cells; Group 2: Re (I) - diselenoether complex group (Re drug group): mice were daily orally treated with Re - diselenoether complex at the dose of 10 mg/kg/24 h for 4 weeks, from day 9 to day 36 after the inoculation of the tumor cells; Group 3: Re (I) - diselenoether complex and CDDP group (combined drug group): mice were daily orally treated with Re-diselenoether complex at the dose of 10 mg/kg/24 h for 4 weeks, from day 9 to day 36 after the inoculation of the tumor cells (as in group 2) and then with CDDP as a single intraperitoneal (IP) injection at a dose of 6 mg/kg on day 41 (as in group 1).
Oral administration of the Re compounds
The Re treatments were started on day 9 after the inoculation of the tumor cells. They were orally administered in the food instead of gavage, as it is a less stressful alternative to oral gavage [11]. Transwean was used to prepare capsules in which the Re (I)-diselenoether was incorporated. The capsules were prepared the day before treatment by mixing 1 g of transwean powder (feed rodent form of powder mixed with water forms a sort of jelly) and 1 mL of water. The mixture was then placed in the wells of a 24-well plate and stored at 4 °C. The next day, the capsules were removed from the mold with a spatula and then cut into small pieces. Re drug at a dose of 10 mg/kg was diluted in a volume of 50 μl and introduced into one of the pieces of “capsule” with a syringe, then that piece was placed in the mouse cage. Once the capsule containing the treatments were consumed normal food pellets were put into the cage until evening. This mode of administration is simple and effective. The treatments were completely consumed with no risk of overdose. Toxicity evaluation. Determination of body weight was performed twice a week for each mouse. Anti-tumor effect . The tumor growth was measured (tumor length, width and volume) twice a week by using an external caliper. The mean tumor volumes [MTV; MTV + (SD); MTV + (SEM)] were estimated. The tumor growth data was recorded for each individually identified mouse. Tumor volume was calculated by using the following formula: V = length x width 2 /2. An imaging by bioluminescence was performed in 2 mice of each group on days 44, 51 and 58 after the inoculation of the tumor cells. The mice were selected to have comparable tumors on day 44.
Statistics
Statistically evaluation of the antitumor effect was assessed by ANOVA test (One way Anova on the ranks).
Results and discussion
Design and synthesis of Re-diselenoether 1
Critical to the antitumor activity of pseudo-symmetric complex 1 was the presence in its framework of a central inorganic core, in which a heavy metal atom (Re) is coordinated with two semi-metal atoms (Se). Although the canonical representation of 1 displayed high molecular symmetry, examination of the 1H NMR spectrum clearly indicated the slow inversion around the two selenium atoms and hence the slow chair-chair interconversion of the six-membered metallacycle abolished the apparent symmetry [8]. The chemical/biological considerations which have governed the design of this three-metal core scaffold were disclosed hereafter. A major interest of Re is related to its very low mammalian toxicity; it has thus been evoked that Re is “one of the least toxic of the metallic elements”. This low toxicity, quite surprising for a heavy metal, can be tentatively interpreted on the basis of its seven degrees of oxidation state (1 to 7) that could authorize subsequent oxidative detoxification processes. Only few data exist on the metabolism of Re compounds. However, a study of the metabolism of [188Re(CO)3(carboxycyclopentadienyl)] in mice revealed the high plasma stability of this Re compound [12]. This study also suggested that the organometallic core of this complex remained unchanged under biological environment. In full agreement with this assertion, the Re compound was essentially excreted as glycine conjugate via the renal route, without further metabolism.
Regarding the presence of two Se atoms in complex 1, it should be mentioned that, fueled by decades of animal studies, Se could significantly reduce the incidence of cancer. This topic is now an area of intense worldwide study [13]. Nevertheless, although inorganic Se has been shown to inhibit carcinogenesis, there is a concern about toxicity, since chronic feeding of inorganic Se (e.g., selenites or selenates) at levels of> 5 ppm is toxic in rodents. However, on the basis of the pioneering work of El-Bayoumy et al. [14–16] and Sanmartin et al. [17–19], a series of synthetic Se compounds have been elaborated, in which the Se atom is connected to two carbon atoms, as in complex 1. These compounds have proved notably more potent and much less toxic than the inorganic counterparts. In contrast to Re compounds, the metabolism of Se compounds in mice is well-documented. It was found that Se compounds which included the RCH2SeCH2R pattern in their structure, such as complex 1, were first cleaved via the trans-selenation pathway into RCH2SeH metabolite that, in turn was converted into H2Se through the β-lyase dealkylation reaction. Both H2Se and CH3SeH are thought to be pivotal metabolites in Se-mediated cancer chemoprevention [7]. A last comment should be made on the design of 1. Since the advantage of all ionic compounds over neutral species is their improved solubility in water, which markedly facilitates their application in biological systems, precursor 2 was ornamented at the Se-levels with two acetic acid moieties [2→4]. At the last step of the synthesis the two carboxylic acid functions were ultimately converted into water-soluble disodium salt 1 (Scheme 1).
The new procedure of synthesis of compound 1 was simple, reproducible, giving a stable product easily authenticated through its IR spectrum. The presence of a d6 fac-[Re(CO)3]+ moiety in complex 1 could explain its high chemical stability. This complex is amphiphilic, soluble in water, and then easy to administer. It also possesses lipophilic properties that allow a facile diffusion across cell membranes and a good biodistribution.
Interaction of Re-diselenoether complex with 9-methylguanine
Extensive studies with many [Re(CO)3] complexes indicate that their cytotoxicity is due to the formation of 1,2-intrastrand adducts e.g., between the N-7 atom of two adjacent guanine residues in DNA, in a fashion similar to cisplatin. Likewise, Re accumulation in cell nucleus treated with Re-diselenoether 1 suggested a possible interaction with nucleic acids [9]. To probe such binding with complex 1, we have investigated the reaction of 5 with 9-methylguanine (9-MeG) as simple surrogate of the guanine base in DNA. Dimethyl ester 5 was used as surrogate of complex 1, since the presence of two sodium carboxylates in latter compound was clearly incompatible with the coupling conditions. Indeed, Alberto and Zobi had previously reported that the [Re(CO)3]+ cation bound to 9-MeG to give mono or bis-adducts [20, 21]. Thus, reaction of 5 with silver fluoroborate gave the corresponding cation which was further reacted with 1 equiv. of 9-MeG in methanol/water mixture. Analysis of the obtained mixture by mass spectrometry indicated that mono-adduct [Re(CO)3(C9H16Se2O4).9-MeG]+ BF4 − (A, Fig. 2) was indeed formed as a minor component (10–15 %). Interestingly, the major product turned out to be the bis-adduct [Re(CO)3(9-MeG)2(H2O)]+ BF4 − (B), previously observed by Alberto and Zobi. The isotopic profiles of both ions are in full agreement with the predicted patterns for the proposed molecular formulas (Fig. 2). In addition, the infrared spectrum of B revealed characteristic CO vibrations at 2027, 1915 and 1895 cm−1 previously reported for this 9-MeG bis-adduct [20]. These observations clearly indicated that the bis-selenoether ligand could be easily displaced by nucleic acid bases to provide guanine bis-adducts, suggesting that the Re-diselenoether complex 1 would be able to form intrastrand lesions. We may hypothesized that in biological medium, following initial aquation, the intermediate Re cation is able to react with nucleic acid bases in nucleus to give mono-adduct (Ion A, Fig. 2; Ion A, Supplementary Fig. 1). The constraint nature of the latter probably facilitated the exchange of the weakly chelating diselenoether ligand by water to give a very reactive ion (Ion D, Supplementary Fig. 1). Finally, a second nucleic acid base addition can easily take place to give 1,2-intrastrand adducts (Ion B, Fig. 2; Ion B, Supplementary Figure 1, whereas the liberated seleno ligand would probably diffuse throughout the cell.
Antitumor effect in vitro of the Re-diselenoether complex 1
It was earlier shown that MCF-7 breast malignant cells were more sensitive to the Re-diselenoether complex 1 than A 549 lung cancer cells and HeLa cervix carcinoma cells [9]. We therefore investigated malignant cells derived from a human breast carcinoma, the hormone-independent MDA-BB231 cells for the morphological and inhibitory effects of the Re-diselenoether complex.
In the presence of a low concentration of Re (I)-diselenoether complex (10 μM), modifications in cell shape and morphology were clearly visible (Fig. 3a) when compared to untreated cells. These cellular alterations corresponded to a reduction in size and a loss of adherence indicating that treated cells were no longer proliferating and possibly included apoptotic cells. Further analysis was performed using Flow cytometry where Forward Scatter (FSC) and Side Scatter (SSC) parameters correspond to measurements of cell size (FSC) and granularity (SSC) (Fig. 3b). Cellular and nuclear debris generated by dead cells were characterized by low FSC/SSC values (<30 K) and excluded from the live gate. Upon 48 h exposure to Re, only 65.1 % of Re-treated cells were identified as alive compared to 92.1 % in the untreated condition. This heterogeneous population included cells of low FSC, indicative of non-proliferative cells, and cells of high SSC, indicative of granular apoptotic cells, which supported the microscopic observations (Fig. 3a).
One of the hallmarks of cancer cells is their dysregulated proliferation. To further evaluate the effect of the Re complex on tumor cell ability to proliferate, MDA-MB231 cells were cultured for 48 h in the presence of 10 μM of Re complex, and for an additional 48 h in Re-free medium. A cell trace violet dye was used to label the cells prior exposure to Re and fluorescence intensity was analysed by flow cytometry (Fig. 4a). Labeled cells at d0 showing maximal fluorescence intensity (shaded red histogram) and unlabeled cells (shaded blue histogram) were used as controls. As expected, untreated MDA-MB231 cells showed cell divisions characterized by a reduction in violet dye fluorescence intensity at d2 and an even greater reduction at day 4 (upper panel). Interestingly, MDA-MB231 cells treated for 48 h with Re showed a reduction in fluorescence intensity at d2, yet to a lesser extent than untreated cells (lower panel). Most importantly, there was no further reduction at d4 indicating no further proliferation. These data show that the Re complex had a negative impact on cell division within the 48 h of culture; this inhibition was not reversed by the absence of Re in the culture beyond 48 h.
a Flow histograms of MDA-MB231 cells untreated or treated with 10 μM Re and analyzed for violet fluorescence intensity at the indicated time points. Plots show histograms of unlabeled control cells (shaded blue), cells labeled with violet dye at d0 prior culture (shaded red) and cells with decreasing amount of violet dye at d2 (green) and d4 (orange) due to a dilution of the violet dye upon cellular division. Data are representatives of 3 independent experiments. b Average fold increase in cell number over 4 days of culture in the presence of 0, 10 or 50 μM of Re complex. Data represents the mean ± SEM values pooled from 2 independent experiments
Cell division was also quantified by standard cell counts at d2 and d4 of culture with 10 and 50 μM of Re complex (Fig. 4b). Exposure to a concentration of 50 μM of Re complex had a striking effect on cell proliferation showing a total inhibition of cell division as early as 48 h which lasted after removal of the Re complex. In line with the flow cytometry experiment (Fig. 4a), the lower concentration of 10 μM of Re-drug was effective to slow down cell proliferation within 48 h of culture and prevented the cells to proliferate further beyond that time point. Altogether, these data show that the Re-diselenoether complex is a potent inhibitor of tumor cell division at low concentration and that this effect is sustained even when the complex is no longer present in the culture.
Potential targets of the Re-diselenoether complex 1
It has been reported that Re-based drugs could target more specifically the malignant cells than the healthy cells [2, 22]. On the other hand, it is noteworthy that some Se-based drugs have demonstrated a selective cytotoxicity against cancerous cells [4–6]. The tumor-specific cytotoxic effects and the cascades of cellular events induced by the major groups of pharmacologically active Se compounds have been reviewed [7]. It is clear that certain redox-active Se compounds induce complex cascades of pro-death signaling at pharmacological concentrations with superior tumor specificity, and that the target molecules are often implicated in drug resistance. This review also emphasized on the chemotherapeutic applications of Se with multi-target attacks on tumor cells, and moreover on the great pharmacological potential of Se for the treatment of resistant cancers.
The Re capture was previously studied in the nucleus of three human breast cancer cells [9]: MCF-7s (sensitive cells), MCF-7R (resistant cells) and MCF-7 MDR (multidrug resistant cells) were exposed to the Re-diselenoether drug at the dose of 400 μM for 48 h (uptake), followed by a post-exposure period of 48 h (efflux). The next intra-nuclear Re concentrations (μM/106 cells) were recorded: MCF-7 s: 0.08 (uptake), 0.25 (efflux), MCF-7R: 0.25 (uptake), 0.12 (efflux) and MCF-7 MDR: 0.15 (uptake), 0.09 (efflux). Regarding the uptake of Re, the concentration in the nucleus was less important in the MCF-7s sensitive cells than in the other cell types. However, in MCF-7R and in MCF-7 MDR, which are MCF-7 cells with an acquired resistance to cytotoxic agents, the nucleus concentration in Re notably decreased after the post-exposure period, indicating an efflux of Re out of the nucleus. This observation is of critical importance regarding the therapeutic protocol. Indeed, with the aim of overcoming the dramatic consequences of a Re efflux in these nuclei, it appeared necessary to maintain a continuous exposure of the malignant cells to the drug; this may be achieved through a daily oral administration.
Antitumor effect of the rhenium-diselenoether 1 in MDA-MB231 tumor-bearing mice
It is known that liposomal rhenium cluster compounds potentiate a platinum-based chemotherapy [22–25]. For that reason, we decided to investigate a potential synergism between cisplatin and the Re-diselenoether complex in our experimental model with transplanted MDA-MB231 Luc+ human breast tumor in mice. Three groups were thus compared: Group 1, treatment by cisplatin (control); group 2, treatment with Re-diselenoether complex; group 3, treatment by Re-diselenoether complex + cisplatin. Re-diselenoether complex showed remarkable antitumor effects versus cisplatin-based chemotherapy in mouse model of breast cancer. The volume of the primitive tumor was remarkably reduced in mice treated with Re-diselenoether complex versus those treated by cisplatin, taken as a control group (p = 0.0006). Results are depicted in Fig. 5 and in Table 1 (Supplementary Table 1).
A first divergence between the three curves was observed on d16, that is to say, 2 weeks after tumor grafting. In group 1 (mice treated with a single administration of cisplatin on d41), a regular tumor volume increase was recorded at the d16-d67 interval, with a final volume reaching up to 140 mm3. Regarding group 2 (mice treated daily with the Re drug from d9 to d36), a plateau was observed at the d16-d44 interval, with a tumor volume not exceeding 20 mm3, followed by a complete regression of the tumors at the d47-67 interval. In group 3 (mice treated with the Re drug according to group 2 protocol, combined with a single administration of cisplatin on d41), the tumor volumes approximately matched those of group 2 until cisplatin administration, followed by a rapid increase until d67, with a final volume reaching up to 180 mm3. These observations deserve the following comments. The absence of any significant inflexion in the profile of curve 1 after cisplatin administration clearly reflected a lack of antitumor activity of this Pt-drug toward MDA-MB231 tumor-bearing mice with this schedule of treatment. By contrast, the profile of curve 2 revealed a nearly immediate antitumor effect of the Re drug, and a complete cure of the tumors after a one-month drug exposure, followed by a post-exposure period of 2 to 3 weeks. Examination of the profile of curve 3 is of peculiar interest regarding the mechanistic aspect of Pt-drugs and Re-drugs. Indeed, to our great surprise, a deleterious effect was observed when Re-drug 1 was co-administered with cisplatin, namely a dramatic collapse of the antitumor activity. This phenomenon can be interpreted on the basis of the binding modes of these metal-based drugs to DNA nucleotides which suggest that a Pt-drug could irreversibly displace a Re-drug from a pre-existing DNA-adduct. This assertion was reinforced through the consideration that both Re-drugs and Pt-drugs target the same recognition sites in DNA bases, exemplified by the N7 center of guanine.
Bioluminescence imaging in mice
The imaging by bioluminescence (Prof. Valérie Rouffiac, Institut Gustave Roussy, France) illustrated the effects of Re (I)-diselenoether complex on the tumor activity (Fig. 6). In the group of the two mice (S1 and S2) treated with the Re drug, the tumor was visible on the first imaging on day 44 after the inoculation of the cancer cells (S1-S2, 11/03 images). On day 51 (18/03), the tumor has disappeared in mouse S2. On day 58 (25/03), there was no detectable tumor in the two mice, indicating a complete regression of the tumor activity, an effect sustained a long time after the interruption of the treatment with the Re-diselenoether complex (end of the treatment on day 36 after the inoculation of the cancer cells).
Incidence of Re-diselenoether complex on pulmonary metastases
The pulmonary metastases could be evaluated in 9 mice of each group (one mouse in each group died before day 42 and the number of pulmonary metastases was not measured). Seven suffering mice were sacrificed on day 65 (two from group 1, one from group 2 and one from group 3: they all had pulmonary metastases). All other mice were sacrificed on day 67, corresponding to the end of the study. Finally, the presence of pulmonary metastases was noted in 9/9 mice in group 1; 5/9 in group 2 and 7/9 in group 3, with a mean number of metastases of 7.22 ± 2.47 in group 1; 3 ± 1.2 in group 2 and 3.57 ± 0.90 in group 3, as represented in Fig. 7. The number of metastases was significantly greater in group 1 versus group 2 (p < 0.05) and in group 1 versus group 3 (p < 0.05), but there was no significant difference in group 2 versus group 3.
Evaluation of the toxicity of Re-diselenoether complex
There was no sign of clinical toxicity in all groups according to the body weight of the mice, as depicted in Fig. 8. There was a death, but one in each group, between days 26 and 39 before the injection of cisplatin, that could be probably attributed to the pulmonary metastases (an autopsy was performed in 2 mice, revealing a great number of metastases). Thus, the dose of 10 mg/kg/24 h of Re-diselenoether appeared to be well-tolerated.
Biodistribution of Re-diselenoether complex in mice
The efficacy of the complex has been established in the animal experiment at a non-toxic dose of 10 mg/kg/d for 4 weeks, both on the primitive tumors and on the metastases. The biodistribution of Re and Se has already been published at this oral dose of 10 mg/kg/d versus 40 mg/kg/d of Re-diselenoether, and it was shown that the oral administration allows a good tissue uptake of Re and Se with a dose-effect [9]. Mice were treated with Re-diselenoether at the dose of 10 mg/kg (corresponding to 3.3 ppm in Re and 2.8 ppm in Se), and 40 mg/kg (13 ppm in Re and 11 ppm in Se), once-a-day for a period of 4 weeks. The distribution study revealed a Re concentration in the liver of 7.1 μmol/kg wet tissue (1.3 ppm) at the dose of 10 mg/kg and 19.8 μmol/kg wet tissue (3.4 ppm) at the dose of 40 mg/kg. Compared to the liver, lower concentrations were recorded in the kidney, namely 4.3 μmol/kg wet tissue (0.8 ppm) at the dose of 10 mg/kg and 8.8 μmol/kg wet tissue (1.6 ppm) at the dose of 40 mg/kg. Regarding Se, the following concentrations (corrected from the essential Se found in the tissues of untreated mice) were recorded : in the liver, 12.9 μmol/kg wet tissue (1.0 ppm) at the dose of 10 mg/kg, 31.1 μmol/kg wet tissue (2.5 ppm) at the dose of 40 mg/kg; in the kidney, 7.0 μmol/kg wet tissue (0.5 ppm) at the dose of 10 mg/kg and 8.8 μmol/kg wet tissue (0.7 ppm) at the dose of 40 mg/kg. These results deserve the following comments. A clear dose-effect of the drug was observed. Indeed, a 4-fold increase of the administered dose of Re-drug 1 (from 10 to 40 mg/kg/d) resulted in a 2.8-fold increase of the Re concentration in the liver and a 2.0-fold increase in the kidney. On the other hand, the Se/Re molar ratios in the liver were 1.8 at the dose of 10 mg/kg and 1.6 at the dose of 40 mg/kg. These ratios are quite close to the 2.0 Se/Re ratio found in the drug (two atoms of Se and one atom of Re per molecule). This observation suggests that the drug might be stored in the liver, more or less as it stands. However, in comparison with the liver, lower Se/Re ratios were recorded in the kidney (1.6 at the dose of 10 mg/kg and 1.0 at the dose of 40 mg/kg). These ratios revealed, as expected, a notable excretion/metabolization of the drug at the kidney level.
Mechanisms of action of diselenoether complex 1
As both Re and Se are well taken up by tissues, it is possible to consider that these two elements will contribute to the antitumor effects by the combination of their different mechanisms of action. The main biological effects of Re are the formation of adducts (single or double strands) with proteins or DNA. These interactions have been extensively studied by Alberto [20, 21, 26] and Zobi [27]. Re can bind to DNA adenine through the N1, N6 positions [28] or to guanine through the N7 position [29, 30], resulting in Re/nucleotide 1:1 or Re/nucleotide 1:2 adducts. Reaction of Re-diselonether 5 with 9-methylguanine used as a simple model of DNA bases clearly established its ability to produced Re/nucleotide 1:1 or Re/nucleotide 1:2 adducts. In contrast to cisplatin, binding of Re drugs to one or two bases is reversible, the Re-adducts having proved less stable than Pt-adducts. In fact, the formation of octahedral Re-adducts may be disfavored since they are generally more bulky and more sterically crowded than square-planar Pt-adducts. The possibility to administer the Re-diselenoether as a continuous oral administration offers an advantage upon cisplatin, which generally needs to be administered through a single injection.
Although the anti-carcinogenic properties of Se are now well established, the modes of action of this element are still a subject of discussion, since they are very complex and not fully understood. However, we can emphasize on the mechanisms of action of Se on redox potential status, inflammation, immunity and cell signaling pathways including the consequences on cell apoptosis, DNA repair and metal detoxification, angiogenesis, metastasis, and finally the effects on the tumor growth.
Among the mechanisms of action of Se, its effects on the oxidative system are perhaps the most important. Se is mainly an anti-oxidant, via the selenoproteins, such as glutathione peroxidase (GPx) and thioredoxine reductase (TrxR). In fact, the existence of a systemic pro-oxidant status in patients with breast cancer is well-established [31], depending on the stage of the disease [32] and on the molecular subtype [33]. A single systemic profile was found in patients with triple negative breast cancer with higher NO levels among subtypes [33]. An other antioxidant, the superoxide dismutase (SOD), has also been proposed to fight against cell proliferation [34]. Ovarian cancer patients resistant to treatments by carboplatin/paclitaxel have a lower level of antioxidant response activation compared to sensitive patients [35], and to restore the oxidative status could increase the efficacy of anticancer cytotoxic drugs. By contrast, the common cytotoxic agents are pro-oxidant drugs, like paclitaxel and doxorubicin [36]. In this respect, high concentrations of Se may produce reactive oxygen species (ROS) and lead to apoptotic cell death by inducing oxidation and cross-linking of protein thiol groups essential for cell survival [37]. According to Jamier et al. [38], Se-based agents could turn the oxidizing redox environment present in certain cancer cells into a lethal cocktail of reactive species, that push these cells over a critical redox threshold and ultimately kill them through apoptosis. This kind of toxicity is highly selective: healthy cells remaining largely unaffected, since changes to their naturally low levels of oxidizing species produce little effect. The balance between pro-oxidative and anti-oxidative effects of Se compounds is still unclear, but it is obvious that the redox potential of cancer cells needs to be taken into account to evaluate the treatments by Se compounds. A very interesting display thiol-proteomics approach to characterize global redox modification of proteins by Se has been proposed by Park et al. [39].
As a second mechanism of action, Se, mainly as selenoproteins, plays an important role in inflammation [40, 41]. There is a strong interaction between inflammation and cancer, and Pt-drugs have even been designed with the aim of targeting NF-kappa B signaling pathways [42]. Se compounds could have an impact on the inflammatory status through the inactivation of NF-kappa B [43, 44]. The interactions between cancer stem cells (CSC) and inflammation are also of a great importance [45] for the development of cancer and its resistance to therapeutic agents. Due to its effect on inflammation, we could expect a role of the Re-diselenoether complex in the growth and activity of CSC.
Studies with Se-compounds indicated that Se may also have positive effects on immune response [46–48], and more specifically on the activity on natural killer (NK) cells [49–51]. Methylselenol, which is the active metabolite of organic Se compounds, has already been shown to regulate the expression of NKG2D ligands by MDA-MB231 and MCF-7 cells [52]. These ligands are involved in the recognition of the malignant cells by NK cells [53–58]. Selenoproteins also mediate T cell immunity through an antioxidant mechanism [59]. Se plays also an important role as an anti-inflammatory agent by tightly regulating the expression of pro-inflammatory genes in immune cells [60].
The role of Se compounds on signaling pathways involved in the development of cancer has become a very attractive area of research. Se-compounds are thought to modulate several kinases. The PI3K/AKT pathway appears as a common target for Se-compounds, but they may modulate different kinases at the same time and their effectiveness depends on the genetic background of the tumor cells [61, 62]. All the Se-compounds did not exhibit kinase inhibitory activity. The type of kinase inhibition greatly depends on the Se derivative. The kinases modulated by S- and Se- derivatives include MAP, ERK, JNK, Akt, Cdc2, Cyclin B1 and Cdc25c amongst others [17]. Therefore, there is a great need for testing the Re-diselenoether complex on different tyrosine and serine/threonine kinases, especially in breast cancer cell lines.
Due to the molecular and biological effects of Se and selenoproteins, there is an expected benefit in cancer patients, not only on the primitive malignant tumor growth, but also on angiogenesis [63, 64] and metastasis [65]. However, the exact schedule of treatment needs to be clarified for each cancer disease, with the help of different markers that remain to be better identified. Plasma Se levels, which have already been investigated in a cohort of breast cancer patients [66], could be useful to monitor the therapy. Whatever the modes of action of these elements, one can argue that there existed a synergistic effect between Re and Se partners accounting for the remarkable, promising antitumor activity of Re(I)- diselenoether complex, already patented in Europe [67].
In summary, Re-diselenoether complex is a promising new metal-based anticancer drug for the treatment of patients with metastatic breast cancer. It proved to efficiently reduce tumor cell division in vitro at a low concentration of 10 μM. It may be orally administered, and the recommended dose is a non-toxic dose of 10 mg/kg/d for a treatment of at least 4 weeks. The efficacy may result from the activity of both Re and Se on key targets of the cancer cells and their microenvironment. Among the mechanisms of action, we confirmed the effects on DNA, due to the Re atom. The effects on the immune system, the redox status, the inflammation and cell signaling pathways attributed to the Se component will be investigated in further studies with models of hormone-independent (MDA-MB231) and hormone-sensitive (MCF-7) metastatic breast cancer.
References
Gasser G, Ott I, Metzler-Nolte N (2011) Organometallic anticancer compounds. J Med Chem 54(1):3–25. doi:10.1021/jm100020w
Leonidova A, Gasser G (2014) Underestimated potential of organometallic rhenium complexes as anticancer agents. ACS Chem Biol 9(10):2180–2193. doi:10.1021/cb500528c
Parson C, Smith V, Krauss C, Banerjee HN, Reilly C, Krause JA, Wachira JM, Giri D, Winstead A, Mandal SK (2014) Anticancer properties of novel rhenium pentylcarbanato compounds against MDA-MB-468(HTB-132) triple node negative human breast cancer cell lines. Br J Pharm Res 4(3):362–367. doi:10.9734/BJPR/2014/4697
Fernandez-Herrera MA, Sandoval-Ramirez J, Sanchez-Sanchez L, Lopez-Munoz H, Escobar-Sanchez ML (2014) Probing the selective antitumor activity of 22-oxo-26-selenocyanocholestane derivatives. Eur J Med Chem 74:451–460. doi:10.1016/j.ejmech.2013.12.059
Guo P, Zhao P, Liu J, Ma H, Bai J, Cao Y, Liu Y, He H, Qi C (2013) Preparation of a novel organoselenium compound and its anticancer effects on cervical cancer cell line HeLa. Biol Trace Elem Res 151(2):301–306. doi:10.1007/s12011-012-9563-x
Ibanez E, Plano D, Font M, Calvo A, Prior C, Palop JA, Sanmartin C (2011) Synthesis and antiproliferative activity of novel symmetrical alkylthio- and alkylseleno-imidocarbamates. Eur J Med Chem 46(1):265–274. doi:10.1016/j.ejmech.2010.11.013
Wallenberg M, Misra S, Bjornstedt M (2014) Selenium cytotoxicity in cancer. Basic Clin Pharmacol 114(5):377–386. doi:10.1111/bcpt.12207
Kermagoret A, Morgant G, D’Angelo J, Tomas A, Roussel P, Bastian G, Collery P, Desmaële D (2011) Synthesis, structural characterization and biological activity against several human tumor cell lines of four rhenium(I) diseleno-ethers complexes: Re(CO)3Cl(PhSe(CH2)2SePh), Re(CO)3Cl(PhSe(CH2)3SePh), Re(CO)3Cl(HO2C–CH2Se(CH2)2SeCH2–CO2H) and Re(CO)3Cl(HO2C–CH2Se(CH2)3SeCH2–CO2H). Polyhedron 30:347–354
Collery P, Bastian G, Santoni S, Mohsen A, Wei M, Collery T, Tomas A, Desmaele D, d’Angelo J (2014) Uptake and efflux of rhenium in cells exposed to rhenium diseleno-ether and tissue distribution of rhenium and selenium after rhenium diseleno-ether treatment in mice. Anticancer Res 34(4):1679–1690
Collery P, Mohsen A, Kermagoret A, d’Angelo J, Morgant G, Desmaele D, Tomas A, Collery T, Wei M, Badawi A (2012) Combination of Three Metals for the Treatment of Cancer: Gallium, Rhenium and Platinum. 1- Determination of the Optimal Schedule of Treatment. Anticancer Res 32(7):2769–2782
Walker MK, Boberg JR, Walsh MT, Wolf V, Trujillo A, Duke MS, Palme R, Felton LA (2012) A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol Appl Pharmacol 260(1):65–69. doi:10.1016/j.taap.2012.01.025
Uehara T, Koike M, Nakata H, Miyamoto S, Motoishi S, Hashimoto K, Oku N, Nakayama M, Arano Y (2003) In vivo recognition of cyclopentadienyltricarbonylrhenium (CpTR) derivatives. Nucl Med Biol 30(3):327–334
Novotny L, Rauko P, Kombian SB, Edafiogho IO (2010) Selenium as a chemoprotective anti-cancer agent: reality or wishful thinking? Neoplasma 57(5):383–391
Desai D, Kaushal N, Gandhi UH, Arner RJ, D’Souza C, Chen G, Vunta H, El-Bayoumy K, Amin S, Prabhu KS (2010) Synthesis and evaluation of the anti-inflammatory properties of selenium-derivatives of celecoxib. Chem Biol Interact 188(3):446–456. doi:10.1016/j.cbi.2010.09.021
Facompre ND, El-Bayoumy K, Sun YW, Pinto JT, Sinha R (2010) 1,4-phenylenebis(methylene)selenocyanate, but not selenomethionine, inhibits androgen receptor and Akt signaling in human prostate cancer cells. Cancer Prev Res (Phila) 3(8):975–984. doi:10.1158/1940-6207.CAPR-10-0054
Facompre ND, Sinha I, El-Bayoumy K, Pinto JT, Sinha R (2012) Remarkable inhibition of mTOR signaling by the combination of rapamycin and 1,4-phenylenebis(methylene)selenocyanate in human prostate cancer cells. Int J Cancer 131(9):2134–2142. doi:10.1002/ijc.27468
Sanmartin C, Plano D, Font M, Palop JA (2011) Kinase regulation by sulfur and selenium containing compounds. Curr Cancer Drug Targets 11(4):496–523
Moreno E, Plano D, Lamberto I, Font M, Encio I, Palop JA, Sanmartin C (2012) Sulfur and selenium derivatives of quinazoline and pyrido[2,3-d]pyrimidine: synthesis and study of their potential cytotoxic activity in vitro. Eur J Med Chem 47(1):283–298. doi:10.1016/j.ejmech.2011.10.056
Sanmartin C, Plano D, Sharma AK, Palop JA (2012) Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci 13(8):9649–9672. doi:10.3390/ijms13089649
Zobi F, Blacque O, Schmalle HW, Spingler B, Alberto R (2004) Head-to-head (HH) and head-to-tail (HT) conformers of cis-bis guanine ligands bound to the [Re(CO)3] + core. Inorg Chem 43(6):2087–2096. doi:10.1021/ic035012a
Zobi F, Spingler B, Alberto R (2005) Guanine and plasmid DNA binding of mono- and trinuclear fac-[Re(CO)3] + complexes with amino acid ligands. Chembiochem 6(8):1397–1405. doi:10.1002/cbic.200400453
Ho J, Lee WY, Koh KJ, Lee PP, Yan YK (2013) Rhenium(I) tricarbonyl complexes of salicylaldehyde semicarbazones: synthesis, crystal structures and cytotoxicity. J Inorg Biochem 119:10–20. doi:10.1016/j.jinorgbio.2012.10.011
Shtemenko N, Collery Ph, Shtemenko A (2006) Synergistic effect of cisplatin and cis-rhenium (III) diadamantate on tumor growth. In: Alpoim M C,Vasconcellos Morais P, Santos M A, Cristovao A J, Centeno J A, Collery P H (ed) Metal Ions in Biology and Medicine John Libbey Eurotext, Paris 9:374–381
Shtemenko N, Collery P, Shtemenko A (2007) Dichlorotetra-μ-isobutyratodirhenium (III): enhancement of cisplatin action and RBC-stabilizing properties. Anticancer Res 27:2487–2492
Shtemenko AV, Collery P, Shtemenko NI, Domasevitch KV, Zabitskaya ED, Golichenko AA (2009) Synthesis, characterization, in vivo antitumor properties of the cluster rhenium compound with GABA ligands and its synergism with cisplatin. Dalton Trans 26:5132–5136. doi:10.1039/b821041a
Zobi F, Blacque O, Sigel RK, Alberto R (2007) Binding interaction of [Re(H2O)3(CO)3] + with the DNA fragment d(CpGpG). Inorg Chem 46(25):10458–10460. doi:10.1021/ic701647m
Zobi F, Spingler B (2012) Post-protein-binding reactivity and modifications of the fac-[Re(CO)3] + core. Inorg Chem 51(3):1210–1212. doi:10.1021/ic2023314
Prater ME, Mindiola DJ, Ouyang X, Dunbar KR (1998) A quadruply-bonded dirhenium complex bridged by two N1/N6 adenate ligands. Inorg Chem Commun 1:475–477
Adams KM, Marzilli LG (2007) fac-[Re(CO)3(H2O)3] + nucleoside monophosphate adducts investigated in aqueous solution by multinuclear NMR spectroscopy. Inorg Chem 46(12):4926–4936. doi:10.1021/ic062410f
Adams KM, Marzilli PA, Marzilli LG (2007) Reactions of fac-[Re(CO)3(H2O)3] + with nucleoside diphosphates and thiamine diphosphate in aqueous solution investigated by multinuclear NMR spectroscopy. Inorg Chem 46(22):9172–9181. doi:10.1021/ic701038f
Mencalha A, Victorino VJ, Cecchini R, Panis C (2014) Mapping oxidative changes in breast cancer: understanding the basic to reach the clinics. Anticancer Res 34(3):1127–1140
Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, Campos FC, Simao AN, Barbosa DS, Pinge-Filho P, Cecchini R, Cecchini AL (2012) Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat 133(3):881–888. doi:10.1007/s10549-011-1851-1
Herrera AC, Panis C, Victorino VJ, Campos FC, Colado-Simao AN, Cecchini AL, Cecchini R (2012) Molecular subtype is determinant on inflammatory status and immunological profile from invasive breast cancer patients. Cancer Immunol Immunother 61(11):2193–2201. doi:10.1007/s00262-012-1283-8
Kim J, Mizokami A, Shin M, Izumi K, Konaka H, Kadono Y, Kitagawa Y, Keller ET, Zhang J, Namiki M (2014) SOD3 acts as a tumor suppressor in PC-3 prostate cancer cells via hydrogen peroxide accumulation. Anticancer Res 34(6):2821–2831
Pons DG, Sastre-Serra J, Nadal-Serrano M, Oliver A, Garcia-Bonafe M, Bover I, Roca P, Oliver J (2012) Initial activation status of the antioxidant response determines sensitivity to carboplatin/paclitaxel treatment of ovarian cancer. Anticancer Res 32(11):4723–4728
Panis C, Herrera AC, Victorino VJ, Campos FC, Freitas LF, De Rossi T, Colado Simao AN, Cecchini AL, Cecchini R (2012) Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res Treat 133(1):89–97. doi:10.1007/s10549-011-1693-x
Lee KH, Jeong D (2012) Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: the selenium paradox (review). Mol Med Rep 5(2):299–304. doi:10.3892/mmr.2011.651
Jamier V, Ba LA, Jacob C (2010) Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chem Eur J 16(36):10920–10928. doi:10.1002/chem.201000884
Park E-M, Choi K-S, Park S-Y, Kong E-S, Zu K, Wu Y, Zhang H, Ip C, Y-M P (2005) A display thiol-proteomics approach to characterize global redox modification of proteins by selenium: implications for the anticancer action of selenium. Cancer Genomics Proteomics 2:25–36
Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16(7):705–743. doi:10.1089/ars.2011.4145
Duntas LH (2009) Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm Metab Res 41(6):443–447. doi:10.1055/s-0029-1220724
Poplawska B, Bielawska A, Surazynski A, Czarnomysy R, Bielawski K (2009) Novel dinuclear platinum(II) complexes targets NFkappaB signaling pathway to induce apoptosis and inhibit metabolism of MCF-7 breast cancer cells. Folia Histochem Cytobiol 47(5):S141–S146. doi:10.2478/v10042-009-0084-1
Kretz-Remy C, Arrigo AP (2001) Selenium: a key element that controls NF-kappa B activation and I kappa B alpha half life. Biofactors 14(1–4):117–125
Youn HS, Lim HJ, Choi YJ, Lee JY, Lee MY, Ryu JH (2008) Selenium suppresses the activation of transcription factor NF-kappa B and IRF3 induced by TLR3 or TLR4 agonists. Int Immunopharmacol 8(3):495–501. doi:10.1016/j.intimp.2007.12.008
Shigdar S, Li Y, Bhattacharya S, O’Connor M, Pu C, Lin J, Wang T, Xiang D, Kong L, Wei MQ, Zhu Y, Zhou S, Duan W (2014) Inflammation and cancer stem cells. Cancer Lett 345(2):271–278. doi:10.1016/j.canlet.2013.07.031
Petrie HT, Klassen LW, Kay HD (1989) Selenium and the immune response: 1. Modulation of alloreactive human lymphocyte functions in vitro. J Leukoc Biol 45(3):207–214
Arthur JR, McKenzie RC, Beckett GJ (2003) Selenium in the immune system. J Nutr 133(5 Suppl 1):1457S–1459S
Broome CS, McArdle F, Kyle JA, Andrews F, Lowe NM, Hart CA, Arthur JR, Jackson MJ (2004) An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 80(1):154–162
Petrie HT, Klassen LW, Klassen PS, O’Dell JR, Kay HD (1989) Selenium and the immune response: 2. Enhancement of murine cytotoxic T-lymphocyte and natural killer cell cytotoxicity in vivo. J Leukoc Biol 45(3):215–220
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1996) Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol Trace Elem Res 52(3):227–239. doi:10.1007/BF02789164
Kiremidjian-Schumacher L, Roy M (1998) Selenium and immune function. Z Ernaehrungswiss 37(Suppl 1):50–56
Hagemann-Jensen M, Uhlenbrock F, Kehlet S, Andresen L, Gabel-Jensen C, Ellgaard L, Gammelgaard B, Skov S (2014) The selenium metabolite methylselenol regulates the expression of ligands that trigger immune activation through the lymphocyte receptor NKG2D. J Biol Chem 289(45):31576–31590. doi:10.1074/jbc.M114.591537
Bae DS, Hwang YK, Lee JK (2012) Importance of NKG2D-NKG2D ligands interaction for cytolytic activity of natural killer cell. Cell Immunol 276(1–2):122–127. doi:10.1016/j.cellimm.2012.04.011
Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ (2003) NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol 4(6):557–564. doi:10.1038/ni929
de Kruijf EM, Sajet A, van Nes JG, Putter H, Smit VT, Eagle RA, Jafferji I, Trowsdale J, Liefers GJ, van de Velde CJ, Kuppen PJ (2012) NKG2D ligand tumor expression and association with clinical outcome in early breast cancer patients: an observational study. BMC Cancer 12:24. doi:10.1186/1471-2407-12-24
Karimi M, Cao TM, Baker JA, Verneris MR, Soares L, Negrin RS (2005) Silencing human NKG2D, DAP10, and DAP12 reduces cytotoxicity of activated CD8+ T cells and NK cells. J Immunol 175(12):7819–7828
Raulet DH, Gasser S, Gowen BG, Deng W, Jung H (2013) Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 31:413–441. doi:10.1146/annurev-immunol-032712-095951
Zafirova B, Wensveen FM, Gulin M, Polic B (2011) Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci 68(21):3519–3529. doi:10.1007/s00018-011-0797-0
Shrimali RK, Irons RD, Carlson BA, Sano Y, Gladyshev VN, Park JM, Hatfield DL (2008) Selenoproteins mediate T cell immunity through an antioxidant mechanism. J Biol Chem 283(29):20181–20185. doi:10.1074/jbc.M802559200
Vunta H, Belda BJ, Arner RJ, Channa Reddy C, Vanden Heuvel JP, Sandeep Prabhu K (2008) Selenium attenuates pro-inflammatory gene expression in macrophages. Mol Nutr Food Res 52(11):1316–1323. doi:10.1002/mnfr.200700346
Plano D, Ibanez E, Calvo A, Palop JA, Sanmartin C (2011) Novel library of selenocompounds as kinase modulators. Molecules 16(8):6349–6364. doi:10.3390/molecules16086349
Ibanez E, Agliano A, Prior C, Nguewa P, Redrado M, Gonzalez-Zubeldia I, Plano D, Palop AJ, Sanmartin C, Calvo A (2012) The quinoline imidoselenocarbamate EI201 blocks the AKT/mTOR pathway and targets cancer stem cells leading to a strong antitumor activity. Curr Med Chem 19(1):3031–3043
Bhattacharya A, Turowski SG, San Martin ID, Rajput A, Rustum YM, Hoffman RM, Seshadri M (2011) Magnetic resonance and fluorescence-protein imaging of the anti-angiogenic and anti-tumor efficacy of selenium in an orthotopic model of human colon cancer. Anticancer Res 31(2):387–393
Li Z, Carrier L, Belame A, Thiyagarajah A, Salvo VA, Burow ME, Rowan BG (2009) Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res Treat 118(1):33–43. doi:10.1007/s10549-008-0216-x
Chen YC, Prabhu KS, Mastro AM (2013) Is selenium a potential treatment for cancer metastasis? Nutrients 5(4):1149–1168. doi:10.3390/nu5041149
Franca CA, Nogueira CR, Ramalho A, Carvalho AC, Vieira SL, Penna AB (2011) Serum levels of selenium in patients with breast cancer before and after treatment of external beam radiotherapy. Ann Oncol 22(5):1109–1112. doi:10.1093/annonc/mdq547
Collery P, D’Angelo J, Morgant G (2015) Rhenium complexes and their pharmaceutical use. Eur Patent 2575800
Acknowledgments
We would like to thank the “Collectivité Territoriale de Corse”, the “Agence de Développement Economique de la Corse”, the “Conseil Général de Haute-Corse”, the “Union Régionale des Praticiens de Santé-Médecins Libéraux de Corse”, the association “Néa” from Corsica, for their great moral and financial support.
Conflict of interest
The authors declare that there are no conflicts of interest. However, Philippe Collery and Jean d’Angelo are designed as co-inventors on the patent on “Rhenium Complexes and their Pharmaceutical Use”. The “Société de Coordination de Recherches Thérapeutiques” is co-owner of the patent together with the Université Paris-Sud and the Centre National de la Recherche Scientifique (CNRS).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOCX 33 kb)
Supplementary Table 1
(DOCX 11 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Collery, P., Mohsen, A., Kermagoret, A. et al. Antitumor activity of a rhenium (I)-diselenoether complex in experimental models of human breast cancer. Invest New Drugs 33, 848–860 (2015). https://doi.org/10.1007/s10637-015-0265-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0265-z