Summary
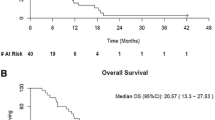
Background There is strong rationale to combine temsirolimus (TEM) with Bevacizumab (BEV) for patients with advanced HCC. Methods A modified two-stage Simon phase II trial was performed with plans to advance to stage 2 if more than 2 patients had confirmed PR or >18 patients were progression free at 6 months out of 25 in stage 1. Toxicity, PFS and overall survival were secondary endpoints. Eligible pts had advanced HCC, Child Pugh A liver status and no prior systemic therapy involving the VEGF or m-TOR targeted agents. Patients were treated with temsirolimus 25 mg IV on Days 1, 8, 15, and 22 of a 28 day cycle and bevacizumab 10 mg/kg IV on Days 1 and 15 of the cycle. Results Twenty-eight eligible patients were enrolled, 26 evaluable receiving a median of 6.5 cycles (range 1–18). Drug related toxicities were common including cytopenias, fatigue, mucositis, diarrhea and mild bleeds. Dose reductions or discontinuation of TEM were common. Accrual closed for presumed futility after interim analysis of the first 25 evaluable patients showed only one PR and 16/25 were progression-free at 6 months. However, the final data update in March 2013 demonstrated 4 confirmed PRs, a 5th unconfirmed PR and 16 /26 progression-free at 6 months. Median PFS and OS were 7 and 14 months respectively. Conclusion This first-line HCC trial evaluating the BEV/TEM doublet reports an ORR of 19 % and OS of 14 months which is favorable but requires further study at a more optimized dose and schedule.

Similar content being viewed by others
References
Thomas MB, Zhu AX (2005) Hepatocellular carcinoma: the need for progress. J Clin Oncol 23(13):2892–9
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340(10):745–50
El-Serag HB, Marrero JA, Rudolph L, Reddy KR (2008) Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 134(6):1752–63
Simonetti RG, Liberati A, Angiolini C et al (1997) Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol 8(2):117–36
Thomas MB, Abbruzzese JL (2005) Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol 23(31):8093–8108
Li X, Tang Z, Zhou G (1998) Expression of vascular endothelial growth factor correlates with invasion and metastatis of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 20:12–4
Llovet JM, Ricci S, Mazzaferro V et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–90
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34
Siegel AB, Cohen EI, Ocean A et al (2008) Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 26(18):2992–8
Lang SA, Moser C, Fichnter-Feigl S et al (2009) Targeting heat-shock protein 90 improves efficacy of rapamycin in a model of hepatocellular carcinoma in mice. Hepatology 49(2):523–32
Huynh H, Chow PK, Palanisamy N et al (2008) Bevacizumab and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Hepatol 49(1):52–60
Zhu AX, Kudo M, Assenat E et al (2014) Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 312(1):57–67
Stephan ES, Datta K, Wang E et al (2004) Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clin Cancer Res 10(20):6993–7000
Merchan JR, Pitot HC, Qin R et al., Final phase II safety and efficacy results of study MC0452: Phase I/II trial of CCI 779 and bevacizumab in advanced renal cell carcinoma. J Clin Oncol 29: 2011 (suppl; abstr 4548)
Sarantopoulous J, Lenz H, LoRusso E et al. Phase I pharmacokinetic study of temsirolimus (CCI-779) in patients with advanced malignancies and normal and impaired liver function : An NCI Organ Dysfunction Working Group Study. J Clin Oncol 29:2011 (suppl abstr 3072)
Thomas MB, Morris JS, Chadha R et al (2009) Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol 27(6):843–850
Hsu CH, Kang YK, Yang TS et al (2013) Bevacizumab with erlotinib as first-line therapy in Asian patients with advanced hepatocellular carcinaom: a multicenter phase II study. Oncology 85(1):44–52
Abou-Alfa GK, Johnson P, Knox JJ et al (2010) Doxorubicin plus sorafenib vs Doxorubicin alone in patients with advanced hepatocellular carcinoma, a randomized trial. JAMA 304(19):2154–2160
Llovet JM, Decaens T, Raoul JL et al (2012) Brivanib versus placebo in patients with advanced hepatocellular carcinoma (HCC) who failed or were intolerant to sorafenib: results from the phase 3 BRISK-PS study. J Hepatol 56(2):S549
Kelley RK, Nimeiri HS, Munster PN et al (2013) Temsirolimus combined with sorafenib in hepatocellulat carcinoma: a phase I dose finding trial with pharmacokinetic and biomarker correlates. Ann Oncol 24(7):1900–7
Acknowledgments
This trial is supported by the NCI. N01-CM-2011-00099 (P2C), N01-CM-2011-00032 (PMH), N01-CM-2011-00100 (Moffitt), N01-CM-2011-00038 (California), N01-CM-2011-00070 (OSU) contracts
Potential conflict of interest
Dr Strosberg: Genentech and Pfizer, limited honoraria.
Dr Saab: Genentech, paid consultant
Dr Knox: Pfizer, research support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knox, J.J., Qin, R., Strosberg, J.R. et al. A phase II trial of bevacizumab plus temsirolimus in patients with advanced hepatocellular carcinoma. Invest New Drugs 33, 241–246 (2015). https://doi.org/10.1007/s10637-014-0169-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0169-3