Summary
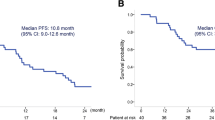
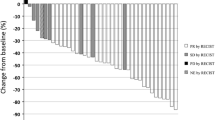
Introduction This study prospectively evaluated the efficacy and safety of pemetrexed and carboplatin followed by maintenance pemetrexed in chemo-naïve patients with advanced nonsquamous non-small cell lung cancer (NSCLC). Methods A total of 109 patients received pemetrexed (500 mg/m2) and carboplatin (area under the curve = 6 mg/mL·min) every 21 days. For patients without disease progression after 4 cycles, pemetrexed was continued until disease progression or unacceptable toxicity. Pre-planned subgroup analysis results based on the presence of epidermal growth factor receptor (EGFR) mutations are also presented. Results The median number of treatment cycles was 5 (range: 1–30) in the entire study period. Most of the grade ≥3 toxicities observed were hematologic in nature, with no increase in the relative incidence associated with continuation maintenance therapy with pemetrexed. Among the 106 total patients assessable for efficacy, the objective response rate was 35.8 %, median progression free survival (PFS) 5.7 months, and median overall survival (OS) 20.2 months. Sixty patients received maintenance pemetrexed (median: 4 cycles, range: 1–26 cycles); median PFS from the beginning of induction treatment was 7.5 months. From the subgroup analysis for EGFR mutation status, the median OS of EGFR wild-type patients (n = 61) was 20.2 months. Conclusions Pemetrexed/carboplatin followed by pemetrexed was well tolerated and active for front-line treatment of advanced nonsquamous NSCLC. Encouraging survival outcomes were observed even in EGFR-wild type patients.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Cojean I, LeChevalier T (1995) Chemotherapy of stage IIIB and IV non-small-cell lung cancer. Ann Oncol 6(Suppl 3):S41–S44
Scagliotti GV, Parikh P, von Pawel J et al (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26:3543–3551
Hirsch FR, Spreafico A, Novello S et al (2008) The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. J Thorac Oncol 3:1468–1481
Einhorn LH (2008) First-line chemotherapy for non-small-cell lung cancer: is there a superior regimen based on histology ? J Clin Oncol 26:3485–3486
Scagliotti G, Brodowicz T, Shepherd FA et al (2011) Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol 6:64–70
Sandler A, Gray R, Perry MC et al (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Patel JD, Bonomi P, Socinski MA et al (2009) Treatment rationale and study design for the pointbreak study: a randomized, open-label phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. Clin Lung Cancer 10:252–256
Sun Y, Ren Y, Fang Z et al (2010) Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 28:4616–4620
Okamoto I, Mitsudomi T, Nakagawa K et al (2010) The emerging role of epidermal growth factor receptor (EGFR) inhibitors in first-line treatment for patients with advanced non-small cell lung cancer positive for EGFR mutations. Ther Adv Med Oncol 2:301–307
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Mok TS, Wu Y-L, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Fukuoka M, Wu YL, Thongprasert S et al (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in asia (IPASS). J Clin Oncol 29:2866–2874
Shaw AT, Yeap BY, Solomon BJ et al (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12:1004–1012
Kwak EL, Bang YJ, Camidge DR et al (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363:1693–1703
Chattopadhyay S, Moran RG, Goldman ID (2007) Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther 6:404–417
Paz-Ares LG, DeMarinis F, Dediu M et al (2012) PARAMOUNT: final overall survival (OS) results of the phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo (plb) plus BSC immediately following induction treatment with pem plus cisplatin (cis) for advanced nonsquamous (NS) non-small cell lung cancer (NSCLC). J Clin Oncol 30(suppl):LBA7507
Sobin LH, Wittekind C (2002) TNM classification of malignant tumours, 6th edn. Wiley-Liss, New York
Zinner RG, Fossella FV, Gladish GW et al (2005) Phase II study of pemetrexed in combination with carboplatin in the first-line treatment of advanced nonsmall cell lung cancer. Cancer 104:2449–2456
Ciuleanu T, Brodowicz T, Zielinski C et al (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374:1432–1440
Zinner RG, Saxman SB, Peng G et al (2010) Treatment rationale and study design for a randomized trial of pemetrexed/carboplatin followed by maintenance pemetrexed versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with advanced non-small-cell lung cancer of nonsquamous histology. Clin Lung Cancer 11:352–357
Acknowledgments
The authors thank all the patients and investigators who participated in this study. This study was sponsored by Eli Lilly Japan K.K.
Disclosure of potential conflicts of interest
Keisuke Aoe, Nobuyuki Yamamoto, Naoyuki Nogami, Terufumi Kato and Kazuhiko Nakagawa received honoraria from Eli Lilly Japan K.K. Immediate family of Terufumi Kato is currently employed by Eli Lilly Japan K.K. Naoto Yoshizuka, Risa Sekiguchi and Kazuhiro Kiyosawa are currently employed by Eli Lilly Japan K.K. Other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is registered with ClinicalTrials.gov. identifier NCT01020786
Funding source: This trial is sponsored by Eli Lilly Japan K.K.
Rights and permissions
About this article
Cite this article
Okamoto, I., Aoe, K., Kato, T. et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naïve patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs 31, 1275–1282 (2013). https://doi.org/10.1007/s10637-013-9941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9941-z