Summary
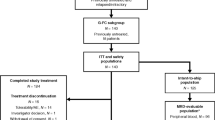
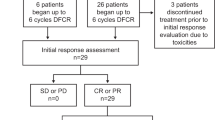
Background Uncontrolled studies comparing pentostatin (P), cyclophosphamide (C), and rituximab (R) (PCR) to fludarabine plus C+R (FCR) suggest similar efficacy with fewer infectious complications with PCR. We compared FCR and PCR in previously-untreated or minimally-treated B-cell chronic lymphocytic leukemia (CLL). Treatment FCR (F 20 mg/m2 Days 1–5, C 600 mg/m2 Day 1, R 375 mg/m2 Day 1) (28-day cycles) or PCR (P 4 mg/m2 Day 1, C 600 mg/m2 Day 1, R 375 mg/m2 Day 1) (21-day cycles). Dose 1 of R: 100 mg/m2 was given on Day 8 Cycle 1 and the remainder on Day 9; in subsequent cycles the entire dose was given on Day 1. Results Ninety-two patients were randomly assigned to each group (N = 184). Groups were balanced; ~20% had received prior chemotherapy. The infection rate (FCR/PCR) was 31%/36%, the infective event rate was 38%/45%; 30 (35%)/37 (44%) patients were hospitalized; total hospitalization days was 271/404. 12 (14%)/6 (7%) patients achieved complete remissions (CR); the overall response rate (ORR) including CR+nodular PR (nPR)+PR was 59%/49%. Grade 3–4 treatment related AEs: neutropenia (69%/57%), leukopenia (34%/17%), thrombocytopenia (13%/6%). Grade 3–4 infections: febrile neutropenia (8%/6%), fever (2%/6%), infection (1%/3%), urinary tract infection (1%/0%), pneumonia (3%/1%), and sepsis (1%/2%); 5 deaths (1 FCR/4 PCR) were treatment-related. Conclusions PCR and FCR have significant activity in CLL and can be given safely in the community setting despite significant toxicity. ORRs were lower than expected; the CR rate was higher (NS) with FCR. This trial did not demonstrate a lower infection rate with PCR.

Similar content being viewed by others
References
Keating MJ, Kantarjian H, O’Brien S, Koller C, Talpaz M, Schachner J et al (1991) Fludarabine: a new agent with marked cytoreductive activity in untreated chronic lymphocytic leukemia. J Clin Oncol 9(1):44–49
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L et al (2000) Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 343(24):1750–1757
Rai KR, Peterson BL, Appelbaum FR, Tallman MS, Belch A, Morrison VA, et al (2000) Long-term survival analysis of the North American Intergroup Study C9011 comparing fludarabine (F) and chlorambucil (C) in previously untreated patients with chronic lymphocytic leukemia (CLL). Blood (ASH Annual Meeting Abstracts) 114:536
O’Brien SM, Kantarjian H, Thomas DA, Giles FJ, Freireich EJ, Cortes J et al (2001) Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol 19(8):2165–2170
Huhn D, von Schilling C, Wilhelm M, Ho AD, Hallek M, Kuse R et al (2001) Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood 98(5):1326–1331
Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE et al (2005) Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 105(1):49–53
Wierda W, O’Brien S, Wen S, Faderl S, Garcia-Manero G, Thomas D et al (2005) Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 23(18):4070–4078
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al (2005) Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 23(18):4079–4088
Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do KA et al (2008) Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 112(4):975–980
Dillman RO, Mick R, McIntyre OR (1998) Pentostatin in chronic lymphocytic leukemia: a phase II trial of Cancer and Leukemia group B. J Clin Oncol 7(4):433–438
Ho AD, Thaler J, Stryckmans P, Coiffier B, Luciani M, Sonneveld P et al (1990) Pentostatin in refractory chronic lymphocytic leukemia: a phase II trial of the European Organization for Research and Treatment of Cancer. Natl Cancer Inst 82(17):1416–1420
Weiss MA, Maslak PG, Jurcic JG, Scheinberg DA, Aliff TB, Lamanna N et al (2003) Pentostatin and cyclophosphamide: an effective new regimen in previously treated patients with chronic lymphocytic leukemia. J Clin Oncol 21(7):1278–1284
Kay NE, Geyer SM, Call TG, Shanafelt TD, Zent CS, Jelinek DF et al (2007) Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 109(2):405–411
Shanafelt TD, Lin T, Geyer SM, Zent CS, Leung N, Kabat B et al (2007) Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer 109(11):2291–2298
Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O’Brien S et al (1996) National Cancer Institute—sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 12:4990–4997
National Cancer Institute’s Cancer Therapy Evaluation Program (NCI CTEP) Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0. URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed August 8, 2009)
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. JASA 53:457–481
Acknowledgments
We thank the patients who shared their experiences with US Oncology physicians (see Appendix), the site coordinators in the field (especially Lisa Mision in Columbia, MO), program manager Julie Boston, RN; project manager Mary Ann Rauch, BS; and data reviewers Tracy Locke, RHIA and Renada Guidry, BS who assured the accuracy and integrity of the data. The authors would especially like to acknowledge R. David Lauper, PharmD, for his support and guidance during the conduct of this study; David passed away as the manuscript was being finalized. He was an excellent colleague and a genuinely nice person. Just after this study was concluded, and during the preparation of the preliminary analysis, Dr. Gary Lee died in a tragic accident. Gary was a superb and caring physician and a great source of inspiration to those who worked with him in his clinic and in the community. David and Gary will be greatly missed.
Manufacturer name
Pentostatin (Nipent ®, Hospira, Inc. [formerly SuperGen, Inc.])
Cyclophosphomide—generic preparations are available and were used at the discretion of the treating physician.
Rituximab (Rituan ®, Genentech/biogen idec)
Fludarabine—generic preparations are available and were used at the discretion of the treating physician.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research support was provided, in part, by Hospira, Inc., Lake Forest, IL
Appendix
Appendix
The following medical oncologists from the US Oncology Research network also participated in this study: Robert J. Belt, Shawnee Mission, KS; Paul D. Richards, Salem, VA; Peter J. Schlegel, Spokane, WA; Raymond Taetle, Tucson, AZ; Patrick V. Acevedo, Ocala, FL; Robert L. Anderson, Waco, TX; Arvind Bhandari, Sugar Land, TX; Ernest W. Cochran Jr., Paris, TX; Philip Y. Dien, Burnsville, MN; David C. Faragher, Aurora, CO; Maria Regina Carrillo Flores, Winter Park/Orlando, FL; Yousuf A. Gaffar, Westminster, MD; Matthew T. Gall, Burnsville, MN; Edward R. George, Norfolk, VA; Timothy K. George, Odessa, TX; Robert H. Gersh, Spokane, WA; Houston E. Holmes, III, Dallas, TX; Pankaj Khandelwal, Odessa, TX; Kathryn S. Kolibaba, Vancouver, WA; Peter X. Lamparello, Latham, NY; Deborah L. Lindquist, Sedona, AZ; Robert L. Marsh, Fairfax, VA; Joseph J. Muscato, Columbia, MO; Rajesh Nahar, Kingston, PA; Sucharu Prakash, Paris, TX; Robert N. Raju, Dayton/Kettering, OH; Michael S. Roberts, Scottsdale, AZ; Steven R. Rousey, Edina, MN; Robert L. Ruxer Jr., Fort Worth, TX; Michael A. Savin, Dallas, TX; Russell C. Tolley, Thornton, CO; Frank T. Ward, Tyler, TX; Ira L. Zackon, Latham, NY; Rony Abou Jawde, St. Joseph, MO; Radhika C. Acharya-Leon, Littleton, CO; Jose M. Acostamadiedo, Elizabeth City, NC; Carlos A. Alemany, Ocoee, FL; Stephen P. Anthony, Spokane, WA; David N. Barrera, Fort Worth, TX; Rebecca E Barrington, Kerrville, TX; Stephen B. Beck, Birmingham, AL; Sridhar Beeram, San Antonio, TX; Maury B. Berger, Ocala, FL; William R. Berry, Raleigh, NC; Anil K.V. Bhogaraju, Lewisville, TX; Michael A. Boxer, Tucson, AZ; Thomas E. Boyd, Yakima, WA; Barry D. Brooks, Dallas, TX; Donald J. Brooks, Minneapolis, MN; Elizabeth E. Campbell, Raleigh, NC; Karen M. Carr, Midland, TX; Ashis K. Chakrabarti, Terre Haute, IN; Benjamin L. Cho, Eugene, OR; Jolanta U. Cichon, Denton, TX; Paul R. Conkling, Norfolk, VA; Linda S. Couch, Longview, TX; Jay G. Courtright, Dallas, TX; John M. Davis II, Lee’s Summit, MO; Yuhoe Gia Dice, San Antonio, TX; Harry G. Dunn, Latham, NY; Charles F. Eisenbeis, Cary, NC; Maha A. Elkordy, Cary, NC; James B. Ellis, San Antonio, TX; William A. Fintel, Salem, VA; Thomas P. Flynn, Minneapolis, MN; Elke K. Friedman, Norfolk, VA; Sandeep S. Gill, Bedford, TX; William L. Gluck, Greenville, SC; Allen Greenberg, Plantation, FL; Manish Gupta, Garland, TX; Elizabeth A Harden, Newport News, VA; James W. Hathorn, Durham, NC; Lanny I. Hecker, Phoenix, AZ; John D. Hunter, Seneca, SC; Sharad K Jain, Denton, TX; John F. Kessler, Newport News, VA; Steven J. Ketchel, Tucson, AZ; Darren M. Kocs, Austin, TX; Peter A. Kovach, Eugene, OR; Flavio Kruter, Owings Mills, MD; Aparna R. Kumar, Tyler, TX; Douglas J. Lee, Seattle, WA; Gary L. Lee, (deceased) Eugene, OR; Jae H. Lee, Eugene, OR; Lixin Liao, Arlington, TX; Keith W. Logie, Fishers, IN; Regan M. Look, Portland, OR; Jose A. Lopez, Fredericksburg, TX; Jeffrey V. Matous, Denver, CO; Kristi J. McIntyre, Dallas, TX; Scott A. McKenney, Beaumont, TX; Richard J. McKittrick, Kansas City, MO; Anton M. Melnyk Jr., Abilene, TX; Mathew Miceli, Ocala, FL; Mohammed K. Nashawaty, Edina, MN; Jairo R. Olivares, Garland, TX; Alvin L. Otsuka, Thornton, CO; Mrugesh P. Patel, Bedford, TX; Kelly B Pendergrass, Kansas City, MO; James B Puckett, Asheville, NC; Syed N. Raza, Abilene, TX; Randy S. Rich, Arlington Hts., IL; Robert M. Rifkin, Denver, CO; Bruce H. Saidman, Kingston, PA; Robert L. Sayre, Colorado Springs, CO; Mark D. Sborov, Edina, MN; John F. Schwerkoske, St. Paul, MN; John E. Seng, Minneapolis, MN; John M. Shaw, Chicago, IL; Mark Sienko, Spokane, WA; Paramjeet Singh, Cary, NC; Mark A. Sitarik, Boulder, CO; David A. Smith, Vancouver, WA; Gary Spitzer, Greenville, SC; Valiant D. Tan, Elizabeth City, NC; Dina J. Tebcherany, Austin, TX; Stephen J. Tremont, Raleigh, NC; Michael C. Trendle, Columbia, MO; Kent A. Tucker, Birmingham, AL; Jeffery C. Ward, Edmonds, WA; Robert S. Wehbie, Raleigh, NC; Eric L. Weinshel, Edina, MN; Charles S. White, III, Dallas, TX; Gary M. Wright, Ocala, FL; Hillary H. Wu, Fishers, IN.
Rights and permissions
About this article
Cite this article
Reynolds, C., Di Bella, N., Lyons, R.M. et al. A Phase III trial of fludarabine, cyclophosphamide, and rituximab vs. pentostatin, cyclophosphamide, and rituximab in B-cell chronic lymphocytic leukemia. Invest New Drugs 30, 1232–1240 (2012). https://doi.org/10.1007/s10637-011-9737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9737-y