Summary
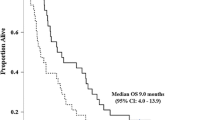
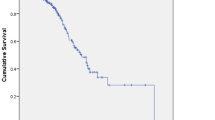
Aim We conducted this phase II study in an effort to evaluate the efficacy and safety of a gemcitabine single chemotherapy as a second-line treatment for biliary tract cancer (BTC) patients who evidenced disease progression after the administration of 5-fluorouracil (5-FU)-based palliative chemotherapy. Patients and Method Patients treated previously with 5-FU-based palliative treatment as a BTC were enrolled in this study. Treatment consisted of gemcitabine at a dosage of 1,250 mg/m2 administered intravenously over a 30-minute period on days 1 and 8 of each 21-day cycle until progression. Results Between Feb. 2006 and July 2009, a total of 32 patients were assigned to treatment groups. 16 patients (50%) had cancers of intra-hepatic cholangiocarcinoma, 12 patients (37.5%) had gall bladder cancer, and 4 patients (12.5%) had extra-hepatic cholangiocarcinoma. In the 29 patients whose tumor responses were evaluated, two achieved a partial response, with an overall response rate of 6.9% (95% confidence interval [CI]: 0.0–16.7%). Six patients (20.7%) evidenced stable disease and 21 patients (72.4%) evidenced progression during the course of treatment. The median follow-up duration was 23.2 months (range: 3.0–53.1 months). The median time to progression (TTP) was 1.6 months (95% CI: 1.3–1.9 months), and the median overall survival (OS) time was 4.1 months (95% CI: 2.7–5.5 months). Poor performance status (ECOG 2) in patients was predictive of shorter TTP. Lower albumin levels (<3.5 g/dL) in patients were predictive of shorter TTP and OS. Conclusions Despite first salvage chemotherapy in the phase II study for patients with 5-FU refractory BTC, the results in terms of RR, TTP, and OS were lower than expected. However, selected patients with good performance status and sufficient albumin levels may have derived some survival benefits from salvage chemotherapy.


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66
Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, Park EC, Ahn YO, Hwang IK, Lee DH, Choi JS, Kim WC, Lee TY, Yoo CI, Bae JM, Kim ON, Chung W, Kong IS, Lee JS (2009) Nationwide cancer incidence in Korea, 2003–2005. Cancer Res Treat 41:122–131
Patel T (2002) Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2:10
Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, Linne T, Svensson C (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7:593–600
Falkson G, MacIntyre JM, Moertel CG (1984) Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer 54:965–969
Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, Fandi L, Zarba J, Armand JP (1998) Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol 9:653–656
Ellis PA, Norman A, Hill A, O’Brien ME, Nicolson M, Hickish T, Cunningham D (1995) Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer 31A:1594–1598
Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, Kim JH, Lee JS, Kang YK (2003) Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol 14:1115–1120
Hong YS, Lee J, Lee SC, Hwang IG, Choi SH, Heo JS, Park JO, Park YS, Lim HY, Kang WK (2007) Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol 60:321–328
Park SH, Park YH, Lee JN, Bang SM, Cho EK, Shin DB, Lee JH (2006) Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer 106:361–365
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C (2004) Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 91:1769–1774
Alberts SR, Al-Khatib H, Mahoney MR, Burgart L, Cera PJ, Flynn PJ, Finch TR, Levitt R, Windschitl HE, Knost JA, Tschetter LK (2005) Gemcitabine, 5-fluorouracil, and leucovorin in advanced biliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group phase II trial. Cancer 103:111–118
Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, Heo JS, Park YS, Kang WK, Park K (2006) A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 106:1339–1346
Lee GW, Kang JH, Kim HG, Lee JS, Jang JS (2006) Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol 29:127–131
Cho JY, Paik YH, Chang YS, Lee SJ, Lee DK, Song SY, Chung JB, Park MS, Yu JS, Yoon DS (2005) Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 104:2753–2758
Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ (2007) Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer 110:1307–1312
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Hezel AF, Zhu AX (2008) Systemic therapy for biliary tract cancers. Oncologist 13:415–423
Lee S, Oh SY, Kim BG, Kwon HC, Kim SH, Rho MH, Kim YH, Rho MS, Jeong JS, Kim HJ (2009) Second-line treatment with a combination of continuous 5-fluorouracil, doxorubicin, and mitomycin-C (conti-FAM) in gemcitabine-pretreated pancreatic and biliary tract cancer. Am J Clin Oncol 32:348–352
Capdevila J, Ramos FJ, Macarulla T, Elez E, Ruiz-Echarri M, Perez-Garcia J, Tabernero J (2009) Development of new drug strategies in infrequent digestive tumors: esophageal, biliary tract, and anal cancers. Curr Opin Oncol 21:374–380
Philip PA, Mahoney MR, Allmer C, Thomas J, Pitot HC, Kim G, Donehower RC, Fitch T, Picus J, Erlichman C (2006) Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 24:3069–3074
Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, Clark JW, Abrams TA, Chan JA, Enzinger PC, Bhargava P, Kwak EL, Allen JN, Jain SR, Stuart K, Horgan K, Sheehan S, Fuchs CS, Ryan DP, Sahani DV (2009) Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol
Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C, Luppi G, Conte P (2010) Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 102:68–72
Acknowledgement
This Paper was supported by the Dong-A University Research Fund.
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST; R13-2002-044-05001-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, S.Y., Jeong, C.Y., Hong, S.C. et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 29, 1066–1072 (2011). https://doi.org/10.1007/s10637-010-9417-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9417-3