Summary
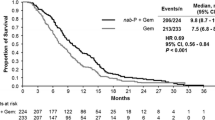
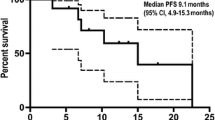
Purpose Gemcitabine (G) is standard therapy for pancreatic cancer. Enzastaurin (E) inhibits PKCβ and PI3K/AKT signaling pathways with a dose-dependent effect on growth of pancreatic carcinoma xenografts. Data suggest that the GE combination may improve clinical outcomes. Methods Primary objective was overall survival (OS); secondary objectives assessed progression-free survival (PFS), response rate (RR), quality of life (QOL), toxicity, and relationships between biomarker expression and clinical outcomes. Patients were randomly assigned (2:1) to GE or G treatment; GE arm: E 500 mg PO daily; loading-dose (1200 mg; Day 1 Cycle 1 only) and G 1000 mg/m2 IV Days 1, 8, and 15 in 28-day cycles; G arm: G as in GE. Biomarker expression was assessed by immunohistochemistry. Results Randomization totaled 130 patients (GE = 86, G = 44); 121 patients were treated (GE = 82, G = 39). GE/G median OS was 5.6/5.1 months; median PFS was 3.4/3.0 months. GE responses: 1 complete response (CR, 1.2%), 6 partial response (PR, 7.4%), and 33 stable disease (SD, 40.7%); disease control rate (DCR=CR+PR+SD, 49.4%). G responses: 2 PR (5.3%) and 16 SD (42.1%); DCR (47.4%). No QOL differences were noted between arms. GE/G Grade 3–4 toxicities included: neutropenia (18.3%/28.2%); thrombocytopenia (14.6%/25.6%); and fatigue (11.0%/7.7%). No statistically significant relationships between biomarker expression and outcomes were observed. However, patients with low expression of cytoplasmic pGSK-3β trended toward greater OS with GE treatment. Conclusions OS, PFS, QOL, and RR were comparable between arms. Adding E to G did not increase hematologic toxicities. GE does not warrant further investigation in unselected pancreatic cancer patients.


Similar content being viewed by others
References
American Cancer Society: Detailed Guide: Pancreatic Cancer. What Are the Key Statistics About Cancer of the Pancreas? URL: http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_pancreatic_cancer_34.asp?sitearea (accessed April 14, 2009)
Berlin JD, Catalano P, Thomas JP et al (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 20:3270–3275
Stathopoulos GP, Syrigos K, Aravantinos G et al (2006) A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer. Br J Cancer 95:587–592
Graff JR, McNulty AM, Hanna KR et al (2005) The protein kinase Cβ-selective inhibitor, enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res 65:7462–7469
Hanauske AR, Oberschmidt O, Hanauske-Abel H et al (2007) Antitumor activity of enzastaurin (LY317615.HCl) against human cancer cells and freshly explanted tumors investigated in in-vitro soft-agar cloning experiments. Invest New Drugs 25:205–210
Spalding AC, Watson R, Davis ME et al (2007) Inhibition of protein kinase Cβ by enzastaurin enhances radiation cytotoxicity in pancreatic cancer cells. Clin Cancer Res 13:6827–6833
Llombart-Cussac A, Matias-Guiu X, Medina DM et al (2009) Enzastaurin inhibits in vivo GSK3β phosphorylation in early breast cancer. American Association of Cancer Research Annual Meeting, Abstract LB-256
Robertson M, Kahl B, Vose J et al (2005) A Phase II study of enzastaurin, a protein kinase C-β (PKCβ) inhibitor, in the treatment of relapsed diffuse large B-cell lymphoma (DLBCL). Blood (ASH Annual Meeting Abstracts) 106: Abstract 934
Fine HA, Kim L, Royce C et al (2005) Results from phase II trial of enzastaurin (LY317615) in patients with recurrent high grade gliomas. J Clin Oncol 23:16s (suppl; abstr 1504)
Oh Y, Herbst RS, Burris H et al (2008) Enzastaurin, an oral serine/threonine kinase inhibitor, as second- or third-line therapy of non-small-cell lung cancer. J Clin Oncol 26:1135–1141
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Eli Lilly and Company—Gemzar® (Gemcitabine HCl for Injection). URL: http://pi.lilly.com/us/gemzar.pdf (accessed 14 April 2009)
National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Publish date 9 August 2006. Available at: http://ctep.cancer.gov/reporting/ctc.html (accessed 20 May 2009)
Kaplan EL, Meier P (1958) Nonparametric estimation of incomplete observations. J Am Stat Assoc 53:457–481
Miller R, Siegmund D (1982) Maximally selected chi-square statistics. Biometrics 38:1011–1016
Spearman C (1904) The proof and measurement of association between two things. Amer J Psychol 15(72–101):1904
Ko AH, Hwang J, Venook AP et al (2005) Serum CA19-9 response as a surrogate for clinical outcome in patients receiving fixed-dose rate gemcitabine for advanced pancreatic cancer. Br J Cancer 93:195–199
Reni M, Cereda S, Balzano G et al (2009) Carbohydrate antigen 19-9 change during chemotherapy for advanced pancreatic adenocarcinoma. Cancer 115:2630–2639
Wong D, Ko AH, Hwang J et al (2008) Serum CA19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas 37:269–274
Cartwright T, Richards DA, Boehm KA (2008) Cancer of the pancreas: are we making any progress? A review of studies in the US Oncology Research Network. Cancer Control 15:308–313
Heinemann V, Quietzsch D, Gieseler F et al (2006) Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24:3946–3952
Herrmann R, Bodoky G, Ruhstaller T et al (2007) Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 25:2212–2217
Oettle H, Richards D, Ramanathan RK et al (2005) A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 16:1639–1645
Richards DA, Oettle H, Vervenne WL et al (2005) Randomized double-blind phase II trial comparing gemcitabine (GEM) plus LY293111 vs. GEM plus placebo in advanced adenocarcinoma of the pancreas. J Clin Oncol 23:16s (suppl; abstr 4092)
Ougolkov AV, Fernandez-Zapico ME, Savoy DN et al (2005) Glycogen synthase kinase-3β participates in nuclear factor κB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res 65:2076–2081
Acknowledgements
We thank our patients, US Oncology physicians (see Appendix), site coordinators (especially Mariel Sumner in Spokane, WA), project managers Staci Bell, Tamika Austin, and Debra Bailey, data reviewer Tracy Locke, and biostatistician Jessica Donato-Jensen. This trial was supported by Lilly USA, LLC.
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported, in part, by a research grant from Lilly USA, LLC; Indianapolis, IN.
Appendix
Appendix
The following oncologists from the USOR network also participated in this study: Harvey, Jimmie H, Birmingham, AL; McKenzie, Barry A, Springfield, OR; Schlegel, Peter J, Spokane, WA; Barrera, David, Fort Worth, TX; Cartwright, Thomas H, Ocala, FL; Esler, Vance William, Amarillo, TX; Flores, Maria Regina C, Winter Park, FL; Flynn, Thomas P, Minneapolis, MN; Hyman, William J, Tyler, TX; Kocs, Darren, Austin, TX; McKenney, Scott A, Beaumont, TX; McMahon, Richard T, Littleton, CO; Negron, Angel G, Fort Worth, TX; Nugent, Francis W, Albany, NY; Obara, Gregory, Las Vegas, NV; Orlowski, Richard, Hickory, NC; Sandbach, John, Austin, TX; Sienko, Mark, Spokane, WA; Tellez, Claudia, Chicago, IL; Acevedo, Patrick V, Ocala, FL; Alemany, Carlos A, Orlando, FL; Aly, Elsayed, Indianapolis, IN; Amare, Mammo, Dallas, TX; Balazs, Andrea, Spokane, WA; Barnett, John, Longmont, CO; Beganovic, Sead, Indianapolis, IN; Berger, Maury B, Ocala, FL; Brandt, Debra Schwab, Torrington, CT; Buchanan, Glenn S, Eugene, OR; Busby, Leslie, Boulder, CO; Chakmakjian, Carl G, Waco, TX; Cichon, Jolanta U, Denton, TX; Cline-Burkhardt, Mika, Las Vegas, NV; Connor, Charles, Plano, TX; Danso, Michael A, Winter Park, FL; DeRosa, William, Morristown, NJ; Di Bella, Nicholas J, Aurora, CO; DiMento, Johanna, Flagstaff, AZ; Encarnacion, Carlos, Waco, TX; Ferris, Linda L, Winfield, IL; Foote, Lawrence E, Sugarland, TX; Forero, Leonardo, Amarillo, TX; Gesme, Dean H, Minneapolis, MN; Goldschmidt, Jerome H Jr, Christiansburg, VA; Gore, Ira Jr, Birmingham, AL; Goslin, Robert H, Amsterdam, NY; Greenfield, Bruce, Santa Fe, NM; Hakimian, David, Niles, IL; Harth, Cheryl A, Dallas, TX; Hellerstedt, Beth, Austin, TX; Howe, Craig WS, St Paul, MN; Hwang, Alice, Portland, OR; Jain, Sharad K, Denton, TX; Jensen, Cynthia L, New Port Richey, FL; Kahn, Michael, Winfield, IL; Kirkpatrick, Haskell, Dallas, TX; Kolibaba, Kathryn S, Vancouver, WA; Kumar, Aparna R, Tyler, TX; Lakhanpal, Shailendra, Birmingham, AL; Lavelle, Joseph W, Dayton/Kettering, OH; Lee†, Gary L, Eugene, OR; Loesch, David M, Indianapolis, IN; Markowitz, Daniel, Edmonds, WA; McCollum, Andrew D, Dallas, TX; Newman, Steven B, Chicago, IL; Overmoyer, Beth A, New Milford, CT; Parikh, Rupesh J, Henderson, NV; Patel, Mrugesh P, Bedford, TX; Raju, Robert N, Dayton/Kettering, OH; Reddy, Chandra, Terre Haute, IN; Reznick, Douglas, Parker, CO; Rotche, Robert M, Christiansburg, VA; Saidman, Bruce, Kingston, PA; Sanatinia, Hamidreza, Las Vegas, NV; Sanchez, James D, Las Vegas, NV; Schlossberg, Howard R, Rexford, NY; Shaffer, David R, Hudson, NY; Snyder, David A, Santa Fe, NM; Solipuram, Praveena R, Thornton, CO; Tebcherany, Dina J, Austin, TX; Tin-U, Caesar K, Sugarland, TX; Tucker, Kent A, Birmingham, AL; Turner, James M, Bedford, TX; Vongkovit, Piyapong, Hickory, NC; Vukelja, Svetislava Judith, Tyler, TX; Wang, Chiyu, Dallas, TX; Wangsness, John A, Maplewood, MN; Ward, Jeffery C, Edmonds, WA; Weissman, Charles H, Latham, NY; Wu, Nini CY, Albany, NY; Zander, Paul J, Minneapolis, MN.
† deceased
Rights and permissions
About this article
Cite this article
Richards, D.A., Kuefler, P.R., Becerra, C. et al. Gemcitabine plus enzastaurin or single-agent gemcitabine in locally advanced or metastatic pancreatic cancer: Results of a Phase II, randomized, noncomparative study. Invest New Drugs 29, 144–153 (2011). https://doi.org/10.1007/s10637-009-9307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9307-8