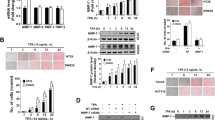
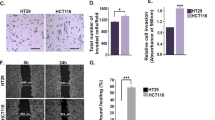
Abstract
Nitric oxide (NO), an uncharged free radical is implicated in various physiological and pathological processes. The present study is an investigation on the effect of NO on proliferation, apoptosis and migration of colon cancer cells. Colon adenocarcinoma cells, WiDr, were used for the in vitro experiments. Tissues from colon adenocarcinoma, adjacent normal and inflammatory tissue and lymph node with metastasis were evaluated for iNOS, MMP-2/9 and Fra-1/Fra-2. NO increases the proliferation of cancer cells and simultaneously prevents apoptosis. Expression of MMP-2/9, RhoB and Rac-1 was enhanced by NO in a time dependent manner. Further, NO increased phosphorylation of ERK1/2 and induced nuclear translocation of Fra-1 and Fra-2. Electrophoretic mobility shift analysis and use of deletion mutant promoter constructs identified role of AP-1 in NO-mediated regulation of MMP-2/9. iNOS, MMP-2/9, Fra-1 and Fra-2 in normal and colon adenocarcinoma tissues were analyzed and it was found that increased expression of these proteins in cancer when compared to normal provides support to our in vitro findings. The study showed that the NO-cGMP-PKG promotes MMP-2/9 expression by activating ERK-1/2 and AP-1. This study reveals the insidious role of NO in imparting tumor aggressiveness.












Similar content being viewed by others
References
Bentz BG, Simmons RL, Haines GK 3rd et al (2000) The yin and yang of nitric oxide: reflections on the physiology and pathophysiology of NO. Head Neck 22(1):71–83
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. N Engl J Med 329(27):2002–2012
Knowles RG, Moncada S (1994) Nitric oxide synthases in mammals. Biochem J 298(Pt 2):249–258
Mocellin S, Bronte V, Nitti D (2007) Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev 27(3):317–352
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95
Hickok JR, Thomas DD (2010) Nitric oxide and cancer therapy: the emperor has NO clothes. Curr Pharm Des 16(4):381–391
Ridnour LA, Thomas DD, Switzer C et al (2008) Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide 19(2):73–76
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8(3):221–233
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295(5564):2387–2392
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2(3):161–174
Tamura T, Nakanishi T, Kimura Y et al (1996) Nitric oxide mediates interleukin-1-induced matrix degradation and basic fibroblast growth factor release in cultured rabbit articular chondrocytes: a possible mechanism of pathological neovascularization in arthritis. Endocrinology 137(9):3729–3737
Orucevic A, Bechberger J, Green AM et al (1999) Nitric-oxide production by murine mammary adenocarcinoma cells promotes tumor-cell invasiveness. Int J Cancer 81(6):889–896
Burlaka AP, Sidorik EP, Ganusevich II et al (2006) Effects of radical oxygen species and NO: formation of intracellular hypoxia and activation of matrix metalloproteinases in tumor tissues. Exp Oncol 28(1):49–53
Zaragoza C, Balbin M, Lopez-Otin C et al (2002) Nitric oxide regulates matrix metalloprotease-13 expression and activity in endothelium. Kidney Int 61(3):804–808
Lopez-Rivera E, Lizarbe TR, Martinez-Moreno M et al (2005) Matrix metalloproteinase 13 mediates nitric oxide activation of endothelial cell migration. Proc Natl Acad Sci USA 102(10):3685–3690
Ridnour LA, Windhausen AN, Isenberg JS et al (2007) Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A 104(43):16898–16903
Marcet-Palacios M, Graham K, Cass C et al (2003) Nitric oxide and cyclic GMP increase the expression of matrix metalloproteinase-9 in vascular smooth muscle. J Pharmacol Exp Ther 307(1):429–436
Bove PF, Wesley UV, Greul AK et al (2007) Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol 36(2):138–146
Kojima H, Nakatsubo N, Kikuchi K et al (1998) Direct evidence of NO production in rat hippocampus and cortex using a new fluorescent indicator: DAF-2 DA. NeuroReport 9(15):3345–3348
Tsikas D, Fuchs I, Gutzki FM et al (1998) Measurement of nitrite and nitrate in plasma, serum and urine of humans by high-performance liquid chromatography, the Griess assay, chemiluminescence and gas chromatography-mass spectrometry: interferences by biogenic amines and N(G)-nitro-l-arginine analogs. J Chromatogr B Biomed Sci Appl 715(2):441–444 (discussion 5-8)
Srinivas P, Gopinath G, Banerji A et al (2004) Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol Carcinog 40(4):201–211
Lancaster JR Jr, Xie K (2006) Tumors face NO problems? Cancer Res 66(13):6459–6462
Du M, Islam MM, Lin L et al (1997) Promotion of proliferation of murine BALB/C3T3 fibroblasts mediated by nitric oxide at lower concentrations. Biochem Mol Biol Int 41(3):625–631
Luczak K, Balcerczyk A, Soszynski M et al (2004) Low concentration of oxidant and nitric oxide donors stimulate proliferation of human endothelial cells in vitro. Cell Biol Int 28(6):483–486
Mei JM, Borchert GL, Donald SP et al (2002) Matrix metalloproteinase(s) mediate(s) NO-induced dissociation of beta-catenin from membrane bound E-cadherin and formation of nuclear beta-catenin/LEF-1 complex. Carcinogenesis 23(12):2119–2122
Mei JM, Hord NG, Winterstein DF et al (2000) Differential formation of beta-catenin/lymphoid enhancer factor-1 DNA binding complex induced by nitric oxide in mouse colonic epithelial cells differing in adenomatous polyposis coli (Apc) genotype. Cancer Res 60(13):3379–3383
Pervin S, Singh R, Hernandez E et al (2007) Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res 67(1):289–299
Mannick JB (2007) Regulation of apoptosis by protein S-nitrosylation. Amino Acids 32(4):523–526
Kim YM, Talanian RV, Billiar TR (1997) Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 272(49):31138–31148
Potter CL, Hanson PJ (2000) Exogenous nitric oxide inhibits apoptosis in guinea pig gastric mucous cells. Gut 46(2):156–162
Pullen NA, Fillmore HL (2010) Induction of matrix metalloproteinase-1 and glioma cell motility by nitric oxide. J Neurooncol 96(2):201–209
Lala PK, Orucevic A (1998) Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer Metastasis Rev 17(1):91–106
McCarthy SM, Bove PF, Matthews DE et al (2008) Nitric oxide regulation of MMP-9 activation and its relationship to modifications of the cysteine switch. Biochemistry 47(21):5832–5840
Harris LK, McCormick J, Cartwright JE et al (2008) S-nitrosylation of proteins at the leading edge of migrating trophoblasts by inducible nitric oxide synthase promotes trophoblast invasion. Exp Cell Res 314(8):1765–1776
Ishii Y, Ogura T, Tatemichi M et al (2003) Induction of matrix metalloproteinase gene transcription by nitric oxide and mechanisms of MMP-1 gene induction in human melanoma cell lines. Int J Cancer 103(2):161–168
Lizarbe TR, Garcia-Rama C, Tarin C et al (2008) Nitric oxide elicits functional MMP-13 protein-tyrosine nitration during wound repair. Faseb J 22(9):3207–3215
Maeda H, Okamoto T, Akaike T (1998) Human matrix metalloprotease activation by insults of bacterial infection involving proteases and free radicals. Biol Chem 379(2):193–200
Rajagopalan S, Meng XP, Ramasamy S et al (1996) Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98(11):2572–2579
Bosca L, Zeini M, Traves PG et al (2005) Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology 208(2):249–258
Shah NS, Billiar TR (1998) Role of nitric oxide in inflammation and tissue injury during endotoxemia and hemorrhagic shock. Environ Health Perspect 106(Suppl 5):1139–1143
Raulo SM, Sorsa T, Tervahartiala T et al (2001) MMP-9 as a marker of inflammation in tracheal epithelial lining fluid (TELF) and in bronchoalveolar fluid (BALF) of COPD horses. Equine Vet J 33(2):128–136
Warner RL, Bhagavathula N, Nerusu KC et al (2004) Matrix metalloproteinases in acute inflammation: induction of MMP-3 and MMP-9 in fibroblasts and epithelial cells following exposure to pro-inflammatory mediators in vitro. Exp Mol Pathol 76(3):189–195
Chen JH, Lin HH, Chiang TA et al (2008) Gaseous nitrogen oxide promotes human lung cancer cell line A549 migration, invasion, and metastasis via iNOS-mediated MMP-2 production. Toxicol Sci 106(2):364–375
Malik MT, Kakar SS (2006) Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2). Mol Cancer 5:61
Jespersen C, Doller A, el Akool S et al (2009) Molecular mechanisms of nitric oxide-dependent inhibition of TPA-induced matrix metalloproteinase-9 (MMP-9) in MCF-7 cells. J Cell Physiol 219(2):276–287
Zaragoza C, Soria E, Lopez E et al (2002) Activation of the mitogen activated protein kinase extracellular signal-regulated kinase 1 and 2 by the nitric oxide-cGMP-cGMP-dependent protein kinase axis regulates the expression of matrix metalloproteinase 13 in vascular endothelial cells. Mol Pharmacol 62(4):927–935
Hovsepian E, Mirkin GA, Penas F et al (2011) Modulation of inflammatory response and parasitism by 15-Deoxy-Delta(12,14) prostaglandin J(2) in Trypanosoma cruzi-infected cardiomyocytes. Int J Parasitol 41(5):553–562
Komalavilas P, Shah PK, Jo H et al (1999) Activation of mitogen-activated protein kinase pathways by cyclic GMP and cyclic GMP-dependent protein kinase in contractile vascular smooth muscle cells. J Biol Chem 274(48):34301–34309
Parri M, Chiarugi P (2010) Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal 8:23
Ridley AJ (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16(10):522–529
Loirand G, Guilluy C, Pacaud P (2006) Regulation of Rho proteins by phosphorylation in the cardiovascular system. Trends Cardiovasc Med 16(6):199–204
Sauzeau V, Rolli-Derkinderen M, Marionneau C et al (2003) RhoA expression is controlled by nitric oxide through cGMP-dependent protein kinase activation. J Biol Chem 278(11):9472–9480
Chen SC, Liu YC, Shyu KG et al (2008) Acute hypoxia to endothelial cells induces activating transcription factor 3 (ATF3) expression that is mediated via nitric oxide. Atherosclerosis 201(2):281–288
Sandau KB, Fandrey J, Brune B (2001) Accumulation of HIF-1alpha under the influence of nitric oxide. Blood 97(4):1009–1015
Brune B, Zhou J (2003) The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1alpha (HIF-1alpha). Curr Med Chem 10(10):845–855
Kim JW, Gao P, Liu YC et al (2007) Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol 27(21):7381–7393
Dulak J, Jozkowicz A (2002) Nitric oxide and angiogenic activity of endothelial cells: direct or VEGF-dependent effect? Cardiovasc Res 56(3):487–488 (author reply 9-91)
Hebert C, Siavash H, Norris K et al (2005) Endostatin inhibits nitric oxide and diminishes VEGF and collagen XVIII in squamous carcinoma cells. Int J Cancer 114(2):195–201
Tian X, Cong M, Zhou W et al (2008) Relationship between protein expression of VEGF-C, MMP-2 and lymph node metastasis in papillary thyroid cancer. J Int Med Res 36(4):699–703
Imler JL, Wasylyk B (1989) AP1, a composite transcription factor implicated in abnormal growth control. Prog Growth Factor Res 1(2):69–77
Leaner VD, Chick JF, Donninger H et al (2009) Inhibition of AP-1 transcriptional activity blocks the migration, invasion, and experimental metastasis of murine osteosarcoma. Am J Pathol 174(1):265–275
Knipp BS, Ailawadi G, Ford JW et al (2004) Increased MMP-9 expression and activity by aortic smooth muscle cells after nitric oxide synthase inhibition is associated with increased nuclear factor-kappaB and activator protein-1 activity. J Surg Res 116(1):70–80
Tkach V, Tulchinsky E, Lukanidin E et al (2003) Role of the Fos family members, c-Fos, Fra-1 and Fra-2, in the regulation of cell motility. Oncogene 22(32):5045–5054
Zhang Q, Kleeberger SR, Reddy SP (2004) DEP-induced fra-1 expression correlates with a distinct activation of AP-1-dependent gene transcription in the lung. Am J Physiol Lung Cell Mol Physiol 286(2):L427–L436
Singh NK, Quyen DV, Kundumani-Sridharan V et al (2010) AP-1 (Fra-1/c-Jun)-mediated induction of expression of matrix metalloproteinase-2 is required for 15S-hydroxyeicosatetraenoic acid-induced angiogenesis. J Biol Chem 285(22):16830–16843
Franchi A, Santucci M, Masini E et al (2002) Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer 95(9):1902–1910
Sun MH, Han XC, Jia MK et al (2005) Expressions of inducible nitric oxide synthase and matrix metalloproteinase-9 and their effects on angiogenesis and progression of hepatocellular carcinoma. World J Gastroenterol 11(38):5931–5937
Acknowledgments
Suboj Babykutty acknowledges Indian Council for Medical Research (ICMR), Government of India, for SRF fellowship and Srinivas Gopala acknowledges Department of Biotechnology (DBT), Government of India, for funds to carry out this work (BT/PR4201/Med/14/513/2004). The authors acknowledge Prof. M. Radhakrishna Pillai, Director, RGCB for extending Flow Cytometry facility; Dr. Kannan S., Professor, RCC for providing support for real-time PCR; Mr. Deepak Roshan V. G. for helping to do the real-time PCR experiments; Mr. Radhakrishnan N. S., Scientific Assistant, Ms Abitha Thomas, for helping in IHC protocol, Mrs. Shirley Stewart, Associate Professor, Mar Ivanios College and Mrs. Nandini R. J., for editing and technical support.
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2012_9464_MOESM2_ESM.jpg
Supplementary Figure 1. Nitrite production estimated using Griess assay. (A) The nitrite level was determined by adding the Griess reagent to the supernatant and incubated as described in materials and methods. After incubation the absorbance was measured at 548 nm. Optical densities were converted into nitrite concentration using recent calibration curve of the NaNO2. Each value is presented as the mean ± SD of determinations from three independent experiments. (B) The protein expression of cyclin D1 levels were expressed as ratio to the expression of β-actin. The above experiment was repeated at least three times. Each value is presented as the mean ± SD of determinations from three independent experiments ***P < 0.001. Real Time RT-PCR analysis of HIF 1-α transcription by NO. (C) The real-time PCR was performed in 7900 HT Fast Real-Time PCR system using MESA green qPCR Mastermix for SYBR Assay. Fold increase of the HIF 1-α was normalized with that of β-actin and was plotted as a graph. Each value is presented as the mean ± SD of determinations from two independent experiments. The mean fold change was significantly higher from the corresponding untreated treated group as analysed by one way ANOVA and Student’s t-test. ***P < 0.001. Flow cytomteric histograms of cell cycle progression (D) and (E). WiDr cells were grown to confluence and treated with or without SNAP (15.6 μM) and then analyzed by FACS as described in materials and methods. The distributions of cells in different phases of the cell cycle are represented in each histogram and figure shown here is the representative histogram from three independent experiments. (JPEG 353 kb)
10585_2012_9464_MOESM3_ESM.jpg
Supplementary Figure 2. Graphical representation of MMPs and RhoGTPase fold change (A and B) The cells were grown in 60 mm dish and cells were exposed to SNAP (15.6 μM) for 4 h and the medium was removed and fresh medium was added and incubated for different time points. After treatment, total RNA was isolated using Pure Link RNA Mini Kit following manufacture’s protocol. RNA was reverse transcribed into cDNA and then amplified by using specific primers by protocols described in the Materials and Methods. Fold change of the target mRNAs (MMPs and Rho GTPases) were normalized with that of β-actin and is plotted as a graph. Each value is presented as the mean ± SD of determinations from two independent experiments. (JPEG 281 kb)
10585_2012_9464_MOESM4_ESM.jpg
Supplementary Figure 3. Graphical representation of TIMP-1/2 fold change (A). After treatment, total RNA was isolated and RNA was reverse transcribed into cDNA and then amplified by using specific primers by protocols described in the Materials and Methods. Fold change of the target mRNAs (TIMPs) were normalized with that of β-actin and is plotted as a graph. Each value is presented as the mean ± SD of determinations from two independent experiments. The mean fold decrease was significantly higher from the corresponding untreated treated group as analysed by Student’s t-test. *P < 0.05; **P < 0.01. Effect of inhibitors on cell viability (B) WiDr cells grown in 96-well plates were treated with or without SNAP(15.6 μM),ODQ(30 μM),KT5823 (180 nm) and PD98059 (10 μM) for 4 h, then the medium was removed and fresh medium was added and incubated for another 48 h. At the end of treatment, cell viability was assessed by MTT assay as described in Materials and Methods. All results were expressed as the mean percentage of control ± S.D. of quadruplicate determinations from three independent experiments. The differences among the mean values were analyzed using 1-way ANOVA followed by Tukey’s post hoc t-test analysis. ***P < 0.001. (JPEG 288 kb)
10585_2012_9464_MOESM5_ESM.jpg
Supplementary Figure 4. Effect of DetaNONOate on viability of WiDr (A) /HUVECs (B). The cells grown in 96-well plates were treated with or without the indicated concentrations of DetaNONOate and at the end of experiment cell viability was assessed by MTT assay as described in Materials and Methods. All results were expressed as the mean percentage over control ± S.D. of quadruplicate determinations from three independent experiments. ***P < 0.001. (JPEG 221 kb)
10585_2012_9464_MOESM6_ESM.tif
Supplementary Figure 5. (A) Detection of endogenous NO production by DAF2-DA. NO levels in colon carcinoma cell line; HCT 116 was detected using DAF-2DA. Cells were incubated with DAF-2-DA and visualized using an inverted fluorescent and light microscope. Images were captured using ProgRes CapturePro v2.8.0. Emission of green fluorescence is an indicative of endogenous NO production. Pictures shown were representative of those that were independently repeated at least two times. (B) Effect of SNAP on viability of colon cancer cells. HCT 116 cells grown in 96-well plates were treated with or without the indicated concentrations of SNAP and at the end of experiment cell viability was assessed by MTT assay as described in Materials and Methods. All results were expressed as the mean percentage of control ± S.D. of quadruplicate determinations from three independent experiments. There was no significant difference between control and SNAP treated HCT cells. (C) NO induced migration of HCT 116 cells. Cells were seeded in 24-well plates and then pre-incubated for 24 h in serum-free DMEM before creating a wound across the cell monolayer with a sterile plastic tip. Cells were allowed to migrate with or without SNAP (15.6 μM). Cell migration into the wound surface was then monitored by microscopy after 24 h and reported as the width of remaining wounded area relative to the initial wounded area. (D) Scratch wound assay was independently repeated two times and the values are plotted as graph. ***P < 0.001. (TIFF 2901 kb)
Rights and permissions
About this article
Cite this article
Babykutty, S., Suboj, P., Srinivas, P. et al. Insidious role of nitric oxide in migration/invasion of colon cancer cells by upregulating MMP-2/9 via activation of cGMP-PKG-ERK signaling pathways. Clin Exp Metastasis 29, 471–492 (2012). https://doi.org/10.1007/s10585-012-9464-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9464-6