Abstract
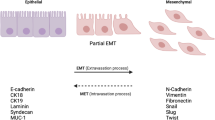
Advanced hepatocellular carcinoma (HCC) is an important cause of cancer mortality. Epithelial-mesenchymal transition (EMT) has been shown to be an important biological process in cancer progression and metastasis. We have focused on elucidating factors that induce EMT to promote carcinogenesis and subsequent metastasis in HCC using the BNL CL.2 (BNL) and BNL 1ME A. 7R.1 (1MEA) cell lines. BNL cells are normal hepatocytes whereas the 1MEA cells are HCC cells derived from chemical transformation of the BNL cells. Their morphological characteristics were examined. Expression levels of hepatocyte growth factor (HGF), markers of EMT and mediators of HGF signaling were determined and functional characteristics were compared. BNL cells were treated with HGF and effects on EMT-marker and mediators of HGF signaling were analyzed. BNL cells display characteristic epithelial morphology whereas 1MEA cells display mesenchymal characteristics. 1MEA cells express and secrete more HGF than BNL cells. There was significantly decreased expression of E-cadherin, albumin, AAT and increased expression of fibronectin, collagen-1, vimentin, snail and slug in 1MEA cells. There was also increased expression of cyclooxygenase-2 (COX-2), Akt and phosphorylated Akt (pAkt) in 1MEA cells. Moreover, 1MEA cells had increased migratory capacity inhibited by inhibition of COX-2 and Akt but not extracellular signal regulated kinase (ERK). Molecular mesenchymal characteristics of 1MEA cells were reversed by inhibition of COX-2, Akt and ERK. Treatment of BNL cells with HGF led to decreased expression of E-cadherin and increased expression of fibronectin, vimentin, snail, slug, COX-2, Akt, pAkt and increased migration, invasiveness and clonogenicity. We conclude that development of HCC is associated with upregulation of HGF which promotes EMT and carcinogenesis via upregulation of COX-2 and Akt. Consequently, HGF signaling may be targeted for therapy in advanced and metastatic HCC.







Similar content being viewed by others
Abbreviations
- COX-2:
-
Cyclooxygenase-2
- ERK:
-
Extracellular signal regulated kinase
- HGF:
-
Hepatocyte growth factor
- DMSO:
-
Dimethyl sulfoxide
References
Bosch FX, Ribes J, Diaz M et al (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127(5):S5–S16
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9(4):265–273
Yang J, Mani SA, Donaher JL et al (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7):927–939
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6):1420–1428
Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796(2):75–90
Kaartinen V, Voncken JW, Shuler C et al (1995) Abnormal lung development and cleft palate in mice lacking TGF-beta-3 indicates defects of epithelial-mesenchymal interaction. Nat Genet 11(4):415–421
Khoury H, Dankort DL, Sadekova S et al (2001) Distinct tyrosine autophosphorylation sites mediate induction of epithelial mesenchymal like transition by an activated ErbB-2/Neu receptor. Oncogene 20(7):788–799
Valles AM, Boyer B, Badet J et al (1990) Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci USA 87(3):1124–1128
Naldini L, Weidner KM, Vigna E et al (1991) Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 10(10):2867–2878
Weidner KM, Hartmann G, Naldini L et al (1993) Molecular characteristics of HGF-SF and its role in cell motility and invasion. EXS 65:311–328
Breuhahn K, Longerich T, Schirmacher P (2006) Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene 25:3787–3800
Zeisberg M, Yang CQ, Martino M et al (2007) Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282(32):23337–23347
Dooley S, Hamzavi J, Ciuclan L et al (2008) Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology 135(2):642–659
Kaimori A, Potter J, Kaimori JY et al (2007) Transforming growth factor-beta 1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem 282(30):22089–22101
Lazarevich N, Cheremnova OA, Varga EV et al (2004) Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology 39(4):1038–1047
Gotzmann J, Fischer ANM, Zojer M et al (2006) A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene 25(22):3170–3185
van Zijl F, Zulehner G, Petz M et al (2009) Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol 5(8):1169–1179
Fischer ANM, Herrera B, Mikula M et al (2005) Integration of Ras subeffector signaling in TGF-beta mediated late stage hepatocarcinogenesis. Carcinogenesis 26(5):931–942
Balaban YH, Us D, Hascelik G et al (2006) Hepatocellular carcinoma and cholangiocarcinoma are associated with high serum levels of hepatocyte growth factor. Indian J Gastroenterol 25(4):223–224
Xie Q, Liu KD, Hu MY et al (2001) SF/HGF-c-Met autocrine and paracrine promote metastasis of hepatocellular carcinoma. World J Gastroenterol 7(6):816–820
Son G, Hirano T, Seki E et al (2006) Blockage of HGF/c-Met system by gene therapy (adenovirus-mediated NK4 gene) suppresses hepatocellular carcinoma in mice. J Hepatol 45(5):688–695
Ding W, You HN, Dang H et al (2010) Epithelial-to-mesenchymal transition of murine liver tumor cells promotes invasion. Hepatology 52(3):945–953
Whittaker S, Marais R, Zhu AX (2010) The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 29(36):4989–5005
Matsumoto K, Nakamura T (1996) Emerging multipotent aspects of hepatocyte growth factor. J Biochem 119(4):591–600
Li XY, Zhan XR, Lu C et al (2010) Mechanisms of hepatocyte growth factor-mediated signaling in differentiation of pancreatic ductal epithelial cells into insulin-producing cells. Biochem Biophys Res Commun 398(3):389–394
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103(2):211–225
Moore AE, Greenhough A, Roberts HR et al (2009) HGF/Met signalling promotes PGE(2) biogenesis via regulation of COX-2 and 15-PGDH expression in colorectal cancer cells. Carcinogenesis 30(10):1796–1804
Abiru S, Nakao K, Ichikawa T et al (2002) Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology 35(5):1117–1124
Gonzales AJ, Goldsworthy TL, Fox TR (1998) Chemical transformation of mouse liver cells results in altered cyclin D CDK protein complexes. Carcinogenesis 19(6):1093–1102
Ogunwobi OA, Beales ILP (2006) Glycine-extended gastrin stimulates proliferation and inhibits apoptosis in colon cancer cells via cyclo-oxygenase-independent pathways. Regul Pept 134(1):1–8
Ogunwobi O, Mutungi G, Beales ILP (2006) Leptin stimulates proliferation and inhibits apoptosis in Barrett’s esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology 147(9):4505–4516
Zhu HZ, Dong HJ, Eksioglu E et al (2007) Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology 133:1649–1659
Behrens J, Mareel MM, Vanroy FM et al (1989) Dissecting tumor cell invasion—epithelial cellsacquireinvasivepropertiesafterthelossofuvomorulin-mediatedcell-cell adhesion. J Cell Biol 108(6):2435–2447
Thompson EW, Torri J, Sabol M et al (1994) Oncogene-induced basement membrane invasiveness in human mammary epithelial cells. Clin Exp Metastasis 12(3):181–194
Guan F, Handa K, Hakomori SI (2009) Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc Natl Acad Sci USA 106(18):7461–7466
Campard D, Lysy PA, Najimi M et al (2008) Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 134(3):833–848
Har CH, Keong CK (2005) Effects of tocotrienols on cell viability and apoptosis in normal murine liver cells (BNL CL.2) and liver cancer cells (BNL 1ME A.7R.1), in vitro. Asia Pac J Clin Nutr 14(4):374–380
Shangguan DH, Meng L, Cao ZHC et al (2008) Identification of liver cancer-specific aptamers using whole live cells. Anal Chem 80(3):721–728
Hsieh HL, Wu CB, Sun CC et al (2006) Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol 207(3):757–766
Acknowledgments
This work was supported by the National Institutes of Health grant R01CA133086 to Chen Liu. Olorunseun O. Ogunwobi is a Postdoctoral Fellow on a NIH T32 grant.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10585_2011_9404_MOESM1_ESM.tif
Supplementary Figure 1 1MEA cells display molecular mesenchymal characteristics in comparison to BNL cells. A, B, D, E. Increased collagen I, vimentin, snail and slug expression respectively in 1MEA cells. C. Decreased AAT expression in 1MEA cells. *P < 0.05; **P < 0.01, N = 3 (TIFF 96 kb)
10585_2011_9404_MOESM2_ESM.tif
Supplementary Figure 2 Assessment of COX-2, ERK and Akt expression and effects of their inhibition on mesenchymal characteristics. Selective inhibitors of Akt (10 μM LY 294002), ERK (25 μM PD 98059) and COX-2 (1 μM celecoxib) inhibit Akt (A), ERK (B) and COX-2 (C). Inhibition of ERK but not Akt also leads to COX-2 inhibition (C). Inhibition of COX-2, Akt and ERK leads to reversal of vimentin (D), collagen I (E) and E-cadherin (F) by 1MEA cells. *P < 0.05, **P < 0.01, N = 3 (TIFF 109 kb)
10585_2011_9404_MOESM3_ESM.tif
Supplementary Figure 3 Migration, clonogenicity and in vivo tumorigenicity. A. 1MEA cells have greater migratory capacity than BNL cells. Selective inhibitors of Akt (10μM LY 294002) and COX-2 (1 μM celecoxib) but not ERK (25 μM PD 98059) inhibited the increased migratory capacity of 1MEA cells. B. 1MEA cells have greater clonogenicity than BNL cells. HGF stimulates increased clonogenicity of BNL cells. C. 1MEA cells but not BNL cells develop tumors in Balb/c mice. *P < 0.05, **P < 0.01, N = 3 (TIFF 876 kb)
Rights and permissions
About this article
Cite this article
Ogunwobi, O.O., Liu, C. Hepatocyte growth factor upregulation promotes carcinogenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via Akt and COX-2 pathways. Clin Exp Metastasis 28, 721–731 (2011). https://doi.org/10.1007/s10585-011-9404-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-011-9404-x