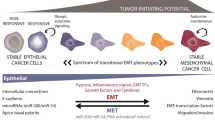
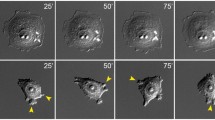
Abstract
The metastatic process, i.e. the dissemination of cancer cells throughout the body to seed secondary tumors at distant sites, requires cancer cells to leave the primary tumor and to acquire migratory and invasive capabilities. In a process of epithelial-mesenchymal transition (EMT), besides changing their adhesive repertoire, cancer cells employ developmental processes to gain migratory and invasive properties that involve a dramatic reorganization of the actin cytoskeleton and the concomitant formation of membrane protrusions required for invasive growth. The molecular processes underlying such cellular changes are still only poorly understood, and the various migratory organelles, including lamellipodia, filopodia, invadopodia and podosomes, still require a better functional and molecular characterization. Notably, direct experimental evidence linking the formation of migratory membrane protrusions and the process of EMT and tumor metastasis is still lacking. In this review, we have summarized recent novel insights into the molecular processes and players underlying EMT on one side and the formation of invasive membrane protrusions on the other side.



Similar content being viewed by others
References
Thiery, J. P., & Sleeman, J. P. (2006). Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol, 7, 131–142.
Grunert, S., Jechlinger, M., & Beug, H. (2003). Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol, 4, 657–665.
Zavadil, J., & Bottinger, E. P. (2005). TGF-beta and epithelial-to-mesenchymal transitions. Oncogene, 24, 5764–5774.
Savagner, P., Yamada, K. M., & Thiery, J. P. (1997). The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol, 137, 1403–1419.
Lo, H. W., Hsu, S. C., Xia, W., Cao, X., Shih, J. Y., & Wei, Y. (2007). Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res, 67, 9066–9076.
Graham, T. R., Zhau, H. E., Odero-Marah, V. A., Osunkoya, A. O., Kimbro, K. S., & Tighiouart, M. (2008). Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res, 68, 2479–2488.
Lee, J. M., Dedhar, S., Kalluri, R., & Thompson, E. W. (2006). The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol, 172(7), 973–981.
Acevedo, V. D., Gangula, R. D., Freeman, K. W., Li, R., Zhang, Y., & Wang, F. (2007). Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition. Cancer Cell, 12, 559–571.
Leong, K. G., Niessen, K., Kulic, I., Raouf, A., Eaves, C., & Pollet, I. (2007). Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med, 204, 2935–2948.
Shintani, Y., Maeda, M., Chaika, N., Johnson, K. R., & Wheelock, M. J. (2008). Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol, 38, 95–104.
Zoltan-Jones, A., Huang, L., Ghatak, S., & Toole, B. P. (2003). Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem, 278, 45801–45810.
Bhowmick, N. A., Ghiassi, M., Bakin, A., Aakre, M., Lundquist, C. A., & Engel, M. E. (2001). Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell, 12, 27–36.
Bakin, A. V., Rinehart, C., Tomlinson, A. K., & Arteaga, C. L. (2002). p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J Cell Sci, 115, 3193–3206.
Janda, E., Lehmann, K., Killisch, I., Jechlinger, M., Herzig, M., & Downward, J. (2002). Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol, 156, 299–313.
Bakin, A. V., Tomlinson, A. K., Bhowmick, N. A., Moses, H. L., & Arteaga, C. L. (2000). Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem, 275, 36803–36810.
Lee, Y. I., Kwon, Y. J., & Joo, C. K. (2004). Integrin-linked kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition. Biochem Biophys Res Commun, 316, 997–1001.
Zavadil, J., Cermak, L., Soto-Nieves, N., & Bottinger, E. P. (2004). Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. EMBO J, 23, 1155–1165.
Gregory, P. A., Bert, A. G., Paterson, E. L., Barry, S. C., Tsykin, A., & Farshid, G. (2008). The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol, 10, 593–601.
Sarrio, D., Rodriguez-Pinilla, S. M., Hardisson, D., Cano, A., Moreno-Bueno, G., & Palacios, J. (2008). Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res, 68, 989–997.
Brabletz, T., Hlubek, F., Spaderna, S., Schmalhofer, O., Hiendlmeyer, E., & Jung, A. (2005). Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs, 179(1–2), 56–65.
Tarin, D., Thompson, E. W., & Newgreen, D. F. (2005). The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res, 65, 5996–6000 discussion 6000-1.
Friedl, P. (2004). Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol, 16, 14–23.
Wicki, A., Lehembre, F., Wick, N., Hantusch, B., Kerjaschki, D., & Christofori, G. (2006). Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell, 9, 261–272.
Yamada, S., Pokutta, S., Drees, F., Weis, W. I., & Nelson, W. J. (2005). Deconstructing the cadherin-catenin-actin complex. Cell, 123, 889–901.
Cavey, M., Rauzi, M., Lenne, P. F., & Lecuit, T. (2008). A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature, 453, 751–756.
Abe, K., & Takeichi, M. (2008). EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A, 105, 13–19.
Stehbens, S. J., Paterson, A. D., Crampton, M. S., Shewan, A. M., Ferguson, C., Akhmanova, A., et al. (2006). Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci, 119(Pt 9), 1801–1811.
Ireton, R. C., Davis, M. A., van Hengel, J., Mariner, D. J., Barnes, K., & Thoreson, M. A. (2002). A novel role for p120 catenin in E-cadherin function. J Cell Biol, 159(3), 465–476.
Davis, M. A., Ireton, R. C., & Reynolds, A. B. (2003). A core function for p120-catenin in cadherin turnover. J Cell Biol, 163, 525–534.
Thoreson, M. A., Anastasiadis, P. Z., Daniel, J. M., Ireton, R. C., Wheelock, M. J., Johnson, K. R., et al. (2000). Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol, 148(1), 189–202.
Wildenberg, G. A., Dohn, M. R., Carnahan, R. H., Davis, M. A., Lobdell, N. A., Settleman, J., et al. (2006). p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell, 127, 1027–1039.
Noren, N. K., Niessen, C. M., Gumbiner, B. M., & Burridge, K. (2001). Cadherin engagement regulates Rho family GTPases. J Biol Chem, 276, 33305–33308.
Noren, N. K., Liu, B. P., Burridge, K., & Kreft, B. (2000). p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol, 150, 567–580.
Comoglio, P. M., Boccaccio, C., & Trusolino, L. (2003). Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol, 15, 565–571.
Chattopadhyay, N., Wang, Z., Ashman, L. K., Brady-Kalnay, S. M., & Kreidberg, J. A. (2003). alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell-cell adhesion. J Cell Biol, 163, 1351–1362.
Vasioukhin, V., Baue, C., Yin, M., & Fuchs, E. (2000). Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell, 100, 209–219.
Shigeta, M., Sanzen, N., Ozawa, M., Gu, J., Hasegawa, H., & Sekiguchi, K. (2003). CD151 regulates epithelial cell-cell adhesion through PKC- and Cdc42-dependent actin cytoskeletal reorganization. J Cell Biol, 163, 165–176.
Helwani, F. M., Kovacs, E. M., Paterson, A. D., Verma, S., Ali, R. G., & Fanning, A. S. (2004). Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol, 164, 899–910.
Canonici, A., Steelant, W., Rigot, V., Khomitch-Baud, A., Boutaghou-Cherid, H., Bruyneel, E., et al. (2008). Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int J Cancer, 122, 572–582.
Reshetnikova, G., Troyanovsky, S., & Rimm, D. L. (2007). Definition of a direct extracellular interaction between Met and E-cadherin. Cell Biol Int, 31, 366–373.
Bissell, M. J., & Radisky, D. (2001). Putting tumours in context. Nat Rev Cancer, 1, 46–54.
Cavallaro, U., & Christofori, G. (2004). Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer, 4, 118–132.
Perl, A. K., Wilgenbus, P., Dahl, U., Semb, H., & Christofori, G. (1998). A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature, 392, 190–193.
Peinado, H., Olmeda, D., & Cano, A. (2007). Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer, 7, 415–428.
Kouzarides, T. (2007). Chromatin modifications and their function. Cell, 128, 693–705.
Jenuwein, T., & Allis, C. D. (2001). Translating the histone code. Science, 293, 1074–1080.
Zhang, Y., & Reinberg, D. (2001). Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev, 15, 2343–2360.
Herranz, N., Pasini, D., Diaz, V. M., Franci, C., Gutierrez, A., & Dave, N. (2008). Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol, 28(15), 4772–4781.
Hou, Z., Peng, H., Ayyanathan, K., Yan, K. P., Langer, E. M., & Longmore, G. D. (2008). The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol Cell Biol, 28, 3198–3207.
Berger, S. L. (2007). The complex language of chromatin regulation during transcription. Nature, 447, 407–412.
Zhu, W., Leber, B., & Andrews, D. W. (2001). Cytoplasmic O-glycosylation prevents cell surface transport of E-cadherin during apoptosis. EMBO J, 20, 5999–6007.
Lochter, A., Galosy, S., Muschler, J., Freedman, N., Werb, Z., & Bissell, M. J. (1997). Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol, 139, 1861–1872.
Marambaud, P., Shioi, J., Serban, G., Georgakopoulos, A., Sarner, S., & Nagy, V. (2002). A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J, 21, 1948–1956.
Maretzky, T., Reiss, K., Ludwig, A., Buchholz, J., Scholz, F., & Proksch, E. (2005). ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A, 102, 9182–9187.
Steinhusen, U., Weiske, J., Badock, V., Tauber, R., Bommert, K., & Huber, O. (2001). Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem, 276, 4972–4980.
Ferber, E. C., Kajita, M., Wadlow, A., Tobiansky, L., Niessen, C., & Ariga, H. (2008). A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem, 283, 12691–12700.
Gumbiner, B. M. (2000). Regulation of cadherin adhesive activity. J Cell Biol, 148, 399–404.
Fujita, Y., Krause, G., Scheffner, M., Zechner, D., Leddy, H. E., & Behrens, J. (2002). Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol, 4, 222–231.
Koenig, A., Mueller, C., Hasel, C., Adler, G., & Menke, A. (2006). Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res, 66, 4662–4671.
Janda, E., Nevolo, M., Lehmann, K., Downward, J., Beug, H., & Grieco, M. (2006). Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene, 25, 7117–7130.
Lu, Z., Ghosh, S., Wang, Z., & Hunter, T. (2003). Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell, 4, 499–515.
Akhtar, N., & Hotchin, N. A. (2001). RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell, 12, 847–862.
Steeg, P. S., Bevilacqua, G., Kopper, L., Thorgeirsson, U. P., Talmadge, J. E., & Liotta, L. A. (1988). Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst, 80, 200–204.
Palacios, F., Schweitzer, J. K., Boshans, R. L., D, , & Souza-Schorey, C. (2002). ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol, 4, 929–936.
Kon, S., Tanabe, K., Watanabe, T., Sabe, H., & Satake, M. (2008). Clathrin dependent endocytosis of E-cadherin is regulated by the Arf6GAP isoform SMAP1. Exp Cell Res, 314, 1415–1428.
Tanabe, K., Torii, T., Natsume, W., Braesch-Andersen, S., Watanabe, T., & Satake, M. (2005). A novel GTPase-activating protein for ARF6 directly interacts with clathrin and regulates clathrin-dependent endocytosis. Mol Biol Cell, 16, 1617–1628.
Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease. Cell, 127, 469–480.
Arce, L., Yokoyama, N. N., & Waterman, M. L. (2006). Diversity of LEF/TCF action in development and disease. Oncogene, 25, 7492–7504.
Wong, N. A., & Pignatelli, M. (2002). Beta-catenin—a linchpin in colorectal carcinogenesis? Am J Pathol, 160, 389–401.
Vignjevic, D., Kojima, S., Aratyn, Y., Danciu, O., Svitkina, T., & Borisy, G. G. (2006). Role of fascin in filopodial protrusion. J Cell Biol, 174, 863–875.
Vignjevic, D., Schoumacher, M., Gavert, N., Janssen, K. P., Jih, G., & Lae, M. (2007). Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res, 67, 6844–6853.
van, Roy, F. M., & McCrea, P. D. (2005). A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer, 5, 956–964.
Nieman, M. T., Prudoff, R. S., Johnson, K. R., & Wheelock, M. J. (1999). N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol, 147, 631–644.
Hulit, J., Suyama, K., Chung, S., Keren, R., Agiostratidou, G., Shan, W., & Dong, X. (2007). N-cadherin signaling potentiates mammary tumor metastasis via enhanced extracellular signal-regulated kinase activation. Cancer Res, 67, 3106–3116.
Gravdal, K., Halvorsen, O. J., Haukaas, S. A., & Akslen, L. A. (2007). A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent iportance for the progress of prostate cancer. Clin Cancer Res, 13, 7003–7011.
Hazan, R. B., Qiao, R., Keren, R., Badano, I., & Suyama, K. (2004). Cadherin switch in tumor progression. Ann N Y Acad Sci, 1014, 155–163.
Shintani, Y., Fukumoto, Y., Chaika, N., Svoboda, R., Wheelock, M. J., & Johnson, K. R. (2008). Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol, 180, 1277–1289.
Alexander, N. R., Tran, N. L., Rekapally, H., Summers, C. E., Glackin, C., & Heimark, R. L. (2006). N-cadherin gene expression in prostate carcinoma is modulated by integrin-dependent nuclear translocation of Twist1. Cancer Res, 66, 3365–3369.
Yang, Z., Zhang, X., Gang, H., Li, X., Li, Z., & Wang, T. (2007). Up-regulation of gastric cancer cell invasion by Twist is accompanied by N-cadherin and fibronectin expression. Biochem Biophys Res Commun, 358, 925–930.
Niu, R. F., Zhang, L., Xi, G. M., Wei, X. Y., Yang, Y., & Shi, Y. R. (2007). Up-regulation of Twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res, 26, 385–394.
Bard, L., Boscher, C., Lambert, M., Mege, R. M., Choquet, D., & Thoumine, O. (2008). A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci, 28, 5879–5890.
El, Sayegh, T. Y., Arora, P. D., Fan, L., Laschinger, C. A., Greer, P. A., & McCulloch, C. A. (2005). Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell, 16, 5514–5527.
Kim, L., & Wong, T. W. (1995). The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol Cell Biol, 15, 4553–4561.
Comunale, F., Causeret, M., Favard, C., Cau, J., Taulet, N., & Charrasse, S. (2007). Rac1 and RhoA GTPases have antagonistic functions during N-cadherin-dependent cell-cell contact formation in C2C12 myoblasts. Biol Cell, 99, 503–517.
Xu, G., Craig, A. W., Greer, P., Miller, M., Anastasiadis, P. Z., & Lilien, J. (2004). Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J Cell Sci, 117, 3207–3219.
Xu, G., Arregui, C., Lilien, J., & Balsamo, J. (2002). PTP1B modulates the association of beta-catenin with N-cadherin through binding to an adjacent and partially overlapping target site. J Biol Chem, 277, 49989–49997.
Theisen, C. S., Wahl 3rd, J. K., Johnson, K. R., & Wheelock, M. J. (2007). NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell, 18, 1220–1232.
Heldin, C. H., Ostman, A., & Ronnstrand, L. (1998). Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta, 1378, F79–113.
Kong, D., Wang, Z., Sarkar, S. H., Li, Y., Banerjee, S., & Saliganan, A. (2008). Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells, 26, 1425–1435.
Sander, E. E., ten Klooster, J. P., van Delft, S., van der Kammen, R. A., & Collard, J. G. (1999). Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol, 147, 1009–1022.
Pertz, O., Hodgson, L., Klemke, R. L., & Hahn, K. M. (2006). Spatiotemporal dynamics of RhoA activity in migrating cells. Nature, 440, 1069–1072.
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D., & Hall, A. (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell, 70, 401–410.
Nimnual, A. S., Taylor, L. J., & Bar-Sagi, D. (2003). Redox-dependent downregulation of Rho by Rac. Nat Cell Biol, 5, 236–241.
Anastasiadis, P. Z., Moon, S. Y., Thoreson, M. A., Mariner, D. J., Crawford, H. C., Zheng, Y., et al. (2000). Inhibition of RhoA by p120 catenin. Nat Cell Biol, 2, 637–644.
Cavallaro, U., Niedermeyer, J., Fuxa, M., & Christofori, G. (2001). N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol, 3, 650–657.
Williams, E. J., Williams, G., Howell, F. V., Skaper, S. D., Walsh, F. S., & Doherty, P. (2001). Identification of an N-cadherin motif that can interact with the fibroblast growth factor receptor and is required for axonal growth. J Biol Chem, 276, 43879–43886.
Hazan, R. B., Phillips, G. R., Qiao, R. F., Norton, L., & Aaronson, S. A. (2000). Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol, 148, 779–790.
Suyama, K., Shapiro, I., Guttman, M., & Hazan, R. B. (2002). A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell, 2, 301–314.
Francavilla, C., Loeffler, S., Piccini, D., Kren, A., Christofori, G., & Cavallaro, U. (2007). Neural cell adhesion molecule regulates the cellular response to fibroblast growth factor. J Cell Sci, 120, 4388–4394.
Sanchez-Heras, E., Howell, F. V., Williams, G., & Doherty, P. (2006). The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J Biol Chem, 281, 35208–35216.
Christofori, G. (2006). New signals from the invasive front. Nature, 441, 444–450.
Marambaud, P., Wen, P. H., Dutt, A., Shioi, J., Takashima, A., & Siman, R. (2003). A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell, 114, 635–645.
Shoval, I., Ludwig, A., & Kalcheim, C. (2007). Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development, 134, 491–501.
Uemura, K., Kihara, T., Kuzuya, A., Okawa, K., Nishimoto, T., Bito, H., & Ninomiya, H. (2006). Activity-dependent regulation of beta-catenin via epsilon-cleavage of N-cadherin. Biochem Biophys Res Commun, 345, 951–958.
Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., dePereda, J. M., et al. (2003). Talin binding to integrin beta tails: a final common step in integrin activation. Science, 302, 103–106.
Deryugina, E. I., Bourdon, M. A., Jungwirth, K., Smith, J. W., & Strongin, A. Y. (2000). Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer, 86, 15–23.
Legate, K. R., Montanez, E., Kudlacek, O., & Fassler, R. (2006). ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol, 7, 20–31.
Mercurio, A. M., & Rabinovitz, I. (2001). Towards a mechanistic understanding of tumor invasion-lessons from the alpha6beta 4 integrin. Semin Cancer Biol, 11, 129–141.
Trusolino, L., Bertotti, A., & Comoglio, P. M. (2001). A signaling adapter function for alpha 6beta 4 integrin in the control of HGF-dependent invasive growth. Cell, 107, 643–654.
Mariotti, A., Kedeshian, P. A., Dans, M., Curatola, A. M., Gagnoux-Palacios, L., & Giancotti, F. G. (2001). EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol, 155, 447–458.
Gambaletta, D., Marchetti, A., Benedetti, L., Mercurio, A. M., Sacchi, A., & Falcioni, R. (2000). Cooperative signaling between alpha (6)beta(4) integrin and ErbB-2 receptor is required to promote phosphatidylinositol 3-kinase-dependent invasion. J Biol Chem, 275, 10604–10610.
Ivaska, J., Reunanen, H., Westermarck, J., Koivisto, L., Kahari, V. M., & Heino, J. (1999). Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J Cell Biol, 147, 401–416.
Ellinger-Ziegelbauer, H., Kelly, K., & Siebenlist, U. (1999). Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol Cell Biol, 19, 3857–3868.
Munger, J. S., Huang, X., Kawakatsu, H., Griffiths, M. J., Dalton, S. L., & Wu, J. (1999). The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell, 96, 319–328.
Mu, D., Cambier, S., Fjellbirkeland, L., Baron, J. L., Munger, J. S., & Kawakatsu, H. (2002). The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol, 157, 493–507.
Wipff, P. J., & Hinz, B. (2008). Integrins and the activation of latent transforming growth factor beta1—An intimate relationship. Eur J Cell Biol, 87(8–9), 601–615.
Haraguchi, M., Okubo, T., Miyashita, Y., Miyamoto, Y., Hayashi, M., & Crotti, T. N. (2008). Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J Biol Chem, 283(35), 23514–23523.
Sharma, M., & Henderson, B. R. (2007). IQ-domain GTPase-activating protein 1 regulates beta-catenin at membrane ruffles and its role in macropinocytosis of N-cadherin and adenomatous polyposis coli. J Biol Chem, 282, 8545–8556.
Ellerbroek, S. M., Wu, Y. I., Overall, C. M., & Stack, M. S. (2001). Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J Biol Chem, 276, 24833–24842.
Wolf, K., Muller, R., Borgmann, S., Brocker, E. B., & Friedl, P. (2003). Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood, 102, 3262–3269.
Cao, J., Chiarelli, C., Richman, O., Zarrabi, K., Kozarekar, P., & Zucker, S. (2008). Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer. J Biol Chem, 283, 6232–6240.
Pulyaeva, H., Bueno, J., Polette, M., Birembaut, P., Sato, H., & Seiki, M. (1997). MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin Exp Metastasis, 15, 111–120.
Bhowmick, N. A., Zent, R., Ghiassi, M., McDonnell, M., & Moses, H. L. (2001). Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem, 276, 46707–46713.
Bravo-Cordero, J. J., Marrero-Diaz, R., Megias, D., Genis, L., Garcia-Grande, A., & Garcia, M. A. (2007). MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J, 26, 1499–1510.
Sheppard, D. (2005). Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev, 24, 395–402.
Roberts, A. B., & Wakefield, L. M. (2003). The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A, 100, 8621–8623.
Bates, R. C. (2005). Colorectal cancer progression: integrin alphavbeta6 and the epithelial-mesenchymal transition (EMT). Cell Cycle, 4, 1350–1352.
Bates, R. C., Bellovin, D. I., Brown, C., Maynard, E., Wu, B., & Kawakatsu, H. (2005). Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest, 115, 339–347.
Araya, J., Cambier, S., Morris, A., Finkbeiner, W., & Nishimura, S. L. (2006). Integrin-mediated transforming growth factor-beta activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol, 169, 405–415.
Li, Y., Dai, C., Wu, C., & Liu, Y. (2007). PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J Am Soc Nephrol, 18, 2534–2543.
Bagnato, A., & Rosano, L. (2007). Epithelial-mesenchymal transition in ovarian cancer progression: a crucial role for the endothelin axis. Cells Tissues Organs, 185, 85–94.
Oloumi, A., McPhee, T., & Dedhar, S. (2004). Regulation of E-cadherin expression and beta-catenin/Tcf transcriptional activity by the integrin-linked kinase. Biochim Biophys Acta, 1691, 1–15.
Etienne-Manneville, S., & Hall, A. (2002). Rho GTPases in cell biology. Nature, 420, 629–635.
Burridge, K. (2004). Wennerberg, K. Rho and Rac take center stage. Cell, 116, 167–179.
Sahai, E., & Marshall, C. J. (2002). RHO-GTPases and cancer. Nat Rev Cancer, 2, 133–142.
Hall, A. (2005). Rho GTPases and the control of cell behaviour. Biochem Soc Trans, 33, 891–895.
Ridley, A. J. (2006). Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol, 16, 522–529.
Lozano, E., Betson, M., & Braga, V. M. (2003). Tumor progression: Small GTPases and loss of cell-cell adhesion. Bioessays, 25, 452–463.
Cozzolino, M., Stagni, V., Spinardi, L., Campioni, N., Fiorentini, C., & Salvati, E. (2003). p120 Catenin is required for growth factor-dependent cell motility and scattering in epithelial cells. Mol Biol Cell, 14, 1964–1977.
Anastasiadis, P. Z. (2007). p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta, 1773, 34–46.
Bellovin, D. I., Bates, R. C., Muzikansky, A., Rimm, D. L., & Mercurio, A. M. (2005). Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res, 65, 10938–10945.
Zondag, G. C., Evers, E. E., ten Klooster, J. P., Janssen, L., van der Kammen, R. A., & Collard, J. G. (2000). Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol, 149, 775–782.
Radisky, D. C., Levy, D. D., Littlepage, L. E., Liu, H., Nelson, C. M., & Fata, J. E. (2005). Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature, 436, 123–127.
Clark, E. A., Golub, T. R., Lander, E. S., & Hynes, R. O. (2000). Genomic analysis of metastasis reveals an essential role for Rock. Nature, 406, 532–535.
Hakem, A., Sanchez-Sweatman, O., You-Ten, A., Duncan, G., Wakeham, A., & Khokha, R. (2005). Rock is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev, 19, 1974–1979.
Nakaya, Y., Sukowati, E. W., Wu, Y., & Sheng, G. (2008). RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol, 10, 765–775.
Hordijk, P. L., ten, Klooster, J. P., van, der, Kammen, R. A., Michiels, F., Oomen, L. C., & Collard, J. G. (1997). Inhibition of invasion of epithelial cells by Tiam1-Rac signaling. Science, 278, 1464–1466.
Malliri, A., van, Es, S., Huveneers, S., & Collard, J. G. (2004). The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem, 279, 30092–30098.
Malliri, A., van der Kammen, R. A., Clark, K., van der Valk, M., Michiels, F., & Collard, J. G. (2002). Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature, 417, 867–871.
Krueger, E. W., Orth, J. D., Cao, H., & McNiven, M. A. (2003). A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell, 14, 1085–1096.
Ballestrem, C., Wehrle-Haller, B., & Imhof, B. A. (1998). Actin dynamics in living mammalian cells. J Cell Sci, 111, 1649–1658.
Suetsugu, S., Yamazaki, D., Kurisu, S., & Takenawa, T. (2003). Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell, 5, 595–609.
Orth, J. D., & McNiven, M. A. (2006). Get off my back! Rapid receptor internalization through circular dorsal ruffles. Cancer Res, 66, 11094–11096.
Vieira, A. V., Lamaze, C., & Schmid, S. L. (1996). Control of EGF receptor signaling by clathrin-mediated endocytosis. Science, 274, 2086–2089.
Dharmawardhane, S., Schurmann, A., Sells, M. A., Chernoff, J., Schmid, S. L., & Bokoch, G. M. (2000). Regulation of macropinocytosis by p21-activated kinase-1. Mol Biol Cell, 11, 3341–3352.
Plattner, R., Kadlec, L., DeMali, K. A., Kazlauskas, A., & Pendergast, A. M. (1999). c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev, 13, 2400–2411.
Yang, Y., Pan, X., Lei, W., Wang, J., Shi, J., Li, F., & Song, J. (2006). Regulation of transforming growth factor-beta 1-induced apoptosis and epithelial-to-mesenchymal transition by protein kinase A and signal transducers and activators of transcription 3. Cancer Res, 66, 8617–8624.
Finn, R. S., Dering, J., Ginther, C., Wilson, C. A., Glaspy, P., & Tchekmedyian, N. (2007). Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat, 105, 319–326.
Srinivasan, D., & Plattner, R. (2006). Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res, 66, 5648–5655.
Watanabe, T., Wang, S., Noritake, J., Sato, K., Fukata, M., & Takefuji, M. (2004). Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell, 7, 871–883.
Etienne-Manneville, S., & Hall, A. (2003). Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature, 421, 753–756.
Sharma, M., Leung, L., Brocardo, M., Henderson, J., Flegg, C., & Henderson, B. R. (2006). Membrane localization of adenomatous polyposis coli protein at cellular protrusions: targeting sequences and regulation by beta-catenin. J Biol Chem, 281, 17140–17149.
Goicoechea, S. M., Arneman, D., & Otey, C. A. (2008). The role of palladin in actin organization and cell motility. Eur J Cell Biol, 87(8–9), 517–525.
Goicoechea, S., Arneman, D., Disanza, A., Garcia-Mata, R., Scita, G., & Otey, C. A. (2006). Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci, 119, 3316–3324.
Ronty, M., Taivainen, A., Heiska, L., Otey, C., Ehler, E., & Song, W. K. (2007). Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res, 313, 2575–2585.
Griffith, O. L., Melck, A., Jones, S. J., & Wiseman, S. M. (2006). Meta-analysis and meta-review of thyroid cancer gene expression profiling studies identifies important diagnostic biomarkers. J Clin Oncol, 24, 5043–5051.
Matoskova, B., Wong, W. T., Salcini, A. E., Pelicci, P. G., & Di, Fiore, P. P. (1995). Constitutive phosphorylation of eps8 in tumor cell lines: relevance to malignant transformation. Mol Cell Biol, 15, 3805–3812.
Yao, J., Weremowicz, S., Feng, B., Gentleman, R. C., Marks, J. R., & Gelman, R. (2006). Combined cDNA array comparative genomic hybridization and serial analysis of gene expression analysis of breast tumor progression. Cancer Res, 66, 4065–4078.
Ryu, B., Jones, J., Hollingsworth, M. A., Hruban, R. H., & Kern, S. E. (2001). Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res, 61, 1833–1838.
Wang, W., Goswami, S., Lapidus, K., Wells, A. L., Wyckoff, J. B., & Sahai, E. (2004). Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res, 64, 8585–8594.
Ronty, M. J., Leivonen, S. K., Hinz, B., Rachlin, A., Otey, C. A., & Kahari, V. M. (2006). Isoform-specific regulation of the actin-organizing protein palladin during TGF-beta1-induced myofibroblast differentiation. J Invest Dermatol, 126, 2387–2396.
Ibarra, N., Pollitt, A., & Insall, R. H. (2005). Regulation of actin assembly by SCAR/WAVE proteins. Biochem Soc Trans, 33, 1243–1246.
LeClainche, C., & Carlier, M. F. (2008). Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev, 88, 489–513.
Innocenti, M., Zucconi, A., Disanza, A., Frittoli, E., Areces, L. B., & Steffen, A. (2004). Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol, 6, 319–327.
Iwaya, K., Norio, K., & Mukai, K. (2007). Coexpression of Arp2 and WAVE2 predicts poor outcome in invasive breast carcinoma. Mod Pathol, 20, 339–343.
Iwaya, K., Oikawa, K., Semba, S., Tsuchiya, B., Mukai, Y., & Otsubo, T. (2007). Correlation between liver metastasis of the colocalization of actin-related protein 2 and 3 complex and WAVE2 in colorectal carcinoma. Cancer Sci, 98, 992–999.
Khoury, H., Dankort, D. L., Sadekova, S., Naujokas, M. A., Muller, W. J., & Park, M. (2001). Distinct tyrosine autophosphorylation sites mediate induction of epithelial mesenchymal like transition by an activated ErbB-2/Neu receptor. Oncogene, 20, 788–799.
Wang, L., Lee, J. F., Lin, C. Y., & Lee, M. J. (2008). Rho GTPases mediated integrin alpha v beta 3 activation in sphingosine-1-phosphate stimulated chemotaxis of endothelial cells. Histochem Cell Biol, 129, 579–588.
Mori, H., Tomari, T., Koshikawa, N., Kajita, M., Itoh, Y., & Sato, H. (2002). CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. EMBO J, 21, 3949–3959.
Coopman, P. J., Do, M. T., Thompson, E. W., & Mueller, S. C. (1998). Phagocytosis of cross-linked gelatin matrix by human breast carcinoma cells correlates with their invasive capacity. Clin Cancer Res, 4, 507–515.
Wang, W., Wyckoff, J. B., Frohlich, V. C., Oleynikov, Y., Huttelmaier, S., & Zavadil, J. (2002). Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res, 62, 6278–6288.
Svitkina, T. M., Bulanova, E. A., Chaga, O. Y., Vignjevic, D. M., Kojima, S., & Vasiliev, J. M. (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol, 160, 409–421.
Pelosi, G., Pastorino, U., Pasini, F., Maissoneuve, P., Fraggetta, F., & Iannucci, A. (2003). Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer, 88, 537–547.
Hashimoto, Y., Shimada, Y., Kawamura, J., Yamasaki, S., & Imamura, M. (2004). The prognostic relevance of fascin expression in human gastric carcinoma. Oncology, 67, 262–270.
Rodriguez-Pinilla, S. M., Sarrio, D., Honrado, E., Hardisson, D., Calero, F., & Benitez, J. (2006). Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res, 12, 1533–1539.
Mongiu, A. K., Weitzke, E. L., Chaga, O. Y., & Borisy, G. G. (2007). Kinetic-structural analysis of neuronal growth cone veil motility. J Cell Sci, 120, 1113–1125.
Saltel, F., Destaing, O., Bard, F., Eichert, D., & Jurdic, P. (2004). Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell, 15, 5231–5241.
Linder, S. (2007). The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol, 17, 107–117.
Linder, S., & Kopp, P. (2005). Podosomes at a glance. J Cell Sci, 118, 2079–2082.
Ayala, I., Baldassarre, M., Caldieri, G., & Buccione, R. (2006). Invadopodia: a guided tour. Eur J Cell Biol, 85, 159–164.
Block, M. R., Badowski, C., Millon-Fremillon, A., Bouvard, D., Bouin, A. P., & Faurobert, E. (2008). Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol, 87(8–9), 491–506.
Kelly, T., Yan, Y., Osborne, R. L., Athota, A. B., Rozypal, T. L., & Colclasure, J. C. (1998). Proteolysis of extracellular matrix by invadopodia facilitates human breast cancer cell invasion and is mediated by matrix metalloproteinases. Clin Exp Metastasis, 16, 501–512.
Tague, S. E., Muralidharan, V., D, , & Souza-Schorey, C. (2004). ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci U S A, 101, 9671–9676.
Artym, V. V., Zhang, Y., Seillier-Moiseiwitsch, F., Yamada, K. M., & Mueller, S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res, 66, 3034–3043.
Angers-Loustau, A., Hering, R., Werbowetski, T. E., Kaplan, D. R., & Del, Maestro, R. F. (2004). SRC regulates actin dynamics and invasion of malignant glial cells in three dimensions. Mol Cancer Res, 2, 595–605.
Clark, E. S., Whigham, A. S., Yarbrough, W. G., & Weaver, A. M. (2007). Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res, 67, 4227–4235.
Yamaguchi, H., Lorenz, M., Kempiak, S., Sarmiento, C., Coniglio, S., & Symons, M. (2005). Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol, 168, 441–452.
Oxmann, D., Held-Feindt, J., Stark, A. M., Hattermann, K., Yoneda, T., & Mentlein, R. (2008). Endoglin expression in metastatic breast cancer cells enhances their invasive phenotype. Oncogene, 27, 3567–3575.
Nakahara, H., Nomizu, M., Akiyama, S. K., Yamada, Y., Yeh, Y., & Chen, W. T. (1996). A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem, 271, 27221–27224.
Wyckoff, J., Wang, W., Lin, E. Y., Wang, Y., Pixley, F., & Stanley, E. R. (2004). A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res, 64, 7022–7029.
Yamaguchi, H., Pixley, F., & Condeelis, J. (2006). Invadopodia and podosomes in tumor invasion. Eur J Cell Biol, 85, 213–218.
Rafii, S., & Lyden, D. (2006). S100 chemokines mediate bookmarking of premetastatic niches. Nat Cell Biol, 8, 1321–1323.
Cortesio, C. L., Chan, K. T., Perrin, B. J., Burton, N. O., Zhang, S., & Zhang, Z. Y. (2008). Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol, 180, 957–971.
Webb, B. A., Jia, L., Eves, R., & Mak, A. S. (2007). Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur J Cell Biol, 86, 189–206.
Bowden, E. T., Onikoyi, E., Slack, R., Myoui, A., Yoneda, T., & Yamada, K. M. (2006). Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res, 312, 1240–1253.
Bharti, S., Inoue, H., Bharti, K., Hirsch, D. S., Nie, Z., & Yoon, H. Y. (2007). Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol, 27, 8271–8283.
Badowski, C., Pawlak, G., Grichine, A., Chabadel, A., Oddou, C., & Jurdic, P. (2008). Paxillin Phosphorylation Controls Invadopodia/Podosomes Spatiotemporal Organization. Mol Biol Cell, 19, 633–645.
Oikawa, T., Itoh, T., & Takenawa, T. (2008). Sequential signals toward podosome formation in NIH-src cells. J Cell Biol, 182(1), 157–169.
Seals, D. F., Azucena Jr., E. F., Pass, I., Tesfay, L., Gordon, R., & Woodrow, M. (2005). The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell, 7, 155–165.
Mueller, S. C., & Chen, W. T. (1991). Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci, 99, 213–225.
Deryugina, E. I., Ratnikov, B., Monosov, E., Postnova, T. I., DiScipio, R., & Smith, J. W. (2001). MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res, 263, 209–223.
Galliher, A. J., & Schiemann, W. P. (2007). Src phosphorylates Tyr284 in TGF-beta type II receptor and regulates TGF-beta stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res, 67, 3752–3758.
Terauchi, M., Kajiyama, H., Yamashita, M., Kato, M., Tsukamoto, H., & Umezu, T. (2007). Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis, 24, 329–339.
Nakahara, H., Mueller, S. C., Nomizu, M., Yamada, Y., Yeh, Y., & Chen, W. T. (1998). Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J Biol Chem, 273, 9–12.
Chuang, Y. Y., Tran, N. L., Rusk, N., Nakada, M., Berens, M. E., & Symons, M. (2004). Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res, 64, 8271–8275.
Sakurai-Yageta, M., Recchi, C., Le, Dez, G., Sibarita, J. B., Daviet, L., & Camonis, J. (2008). The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol, 181, 985–998.
Buccione, R., Orth, J. D., & McNiven, M. A. (2004). Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol, 5, 647–657.
Gimona, M., Buccione, R., Courtneidge, S. A., & Linder, S. (2008). Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol, 20, 235–241.
Vignjevic, D., & Montagnac, G. (2008). Reorganisation of the dendritic actin network during cancer cell migration and invasion. Semin Cancer Biol, 18, 12–22.
Weaver, A. M. (2008). Invadopodia. Curr Biol, 18, 362–364.
Varon, C., Tatin, F., Moreau, V., Van Obberghen-Schilling, E., Fernandez-Sauze, S., Reuzeau, E., et al. (2006). Transforming growth factor beta induces rosettes of podosomes in primary aortic endothelial cells. Mol Cell Biol, 26, 3582–3594.
Frame, M. C. (2004). Newest findings on the oldest oncogene; how activated src does it. J Cell Sci, 117, 989–998.
Xie, L., Law, B. K., Aakre, M. E., Edgerton, M., Shyr, Y., Bhowmick, N. A., et al. (2003). Transforming growth factor beta-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res, 5, S187–198.
Fonsatti, E., Altomonte, M., Nicotra, M. R., Natali, P. G., & Maio, M. (2003). Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene, 22, 6557–6563.
Mercado-Pimentel, M. E., Hubbard, A. D., & Runyan, R. B. (2007). Endoglin and Alk5 regulate epithelial-mesenchymal transformation during cardiac valve formation. Dev Biol, 304, 420–432.
Lua, B. L., & Low, B. C. (2004). BPGAP1 interacts with cortactin and facilitates its translocation to cell periphery for enhanced cell migration. Mol Biol Cell, 15, 2873–2883.
Head, J. A., Jiang, D., Li, M., Zorn, L. J., Schaefer, E. M., Parsons, J. T., & Weed, S. A. (2003). Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell, 14, 3216–3229.
Lee, S. H. (2005). Interaction of nonreceptor tyrosine-kinase Fer and p120 catenin is involved in neuronal polarization. Mol Cells, 20, 256–262.
Acknowledgements
We apologize to all colleagues whose important work we could not cite due to space restrictions. Research in the laboratory of the authors has been supported by the EU-FP6 framework program BRECOSM LSHC-CT-2004-503224, the EU-FP7 framework program TUMIC 2008-201662, the NCCR Molecular Oncology of the Swiss National Science Foundation, the Swiss Cancer League, and the Krebsliga Beider Basel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yilmaz, M., Christofori, G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 28, 15–33 (2009). https://doi.org/10.1007/s10555-008-9169-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9169-0