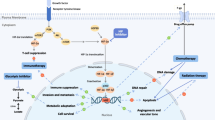
Abstract
Hypoxia, a decrease in oxygen levels, is a hallmark of solid tumors. Hypoxic cells are more resistant to killing by ionizing radiation and chemotherapy, are more invasive and metastatic, resistant to apoptosis, and genetically unstable. Over the last two decades, the discovery of Hypoxia Inducible Factors, a family of transcription factors crucially involved in the response of mammalian cells to oxygen deprivation, has led to the identification of a molecular target associated with hypoxia suitable for the development of cancer therapeutics. These features of solid tumors may offer a unique opportunity for selective therapeutic approaches. A number of strategies targeting hypoxia and/or Hypoxia Inducible Factors (HIF) have been developed over the last several years and will be described. The exponentially growing interest in therapeutic strategies targeting hypoxia/HIF will undoubtedly generate more active compounds for preclinical and clinical development. A rational development plan aimed to validate target inhibition in preclinical models and early clinical trials is essential for a rapid translation of these agents to the treatment of human cancers.
Similar content being viewed by others
References
Harris, A. L. (2002). Hypoxia—A key regulatory factor in tumour growth. Nature Reviews. Cancer, 2, 38–47.
Brown, J. M, & Wilson, W. R. (2004). Exploiting tumour hypoxia in cancer treatment. Nature Reviews. Cancer, 4, 437–447.
Melillo, G. (2006). Inhibiting hypoxia-inducible factor 1 for cancer therapy. Molecular Cancer Research, 4, 601–605.
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nature Reviews. Cancer, 3, 721–732.
Giaccia, A., Siim, B. G., & Johnson, R. S. (2003). HIF-1 as a target for drug development. Nature Reviews. Drug Discovery, 2, 803–811.
Maxwell, P. H. (2005). The HIF pathway in cancer. Seminars in Cell & Developmental Biology, 16, 523–530.
Melillo, G. (2004). HIF-1: A target for cancer, ischemia and inflammation—Too good to be true? Cell Cycle, 3, 154–155.
Kaufman, B., Scharf, O., Arbeit, J., Ashcroft, M., Brown, J. M., Bruick, R. K., et al. (2004). Proceedings of the oxygen homeostasis/hypoxia meeting. Cancer Research, 64, 3350–3356.
Melillo, G., & Semenza, G. L. (2006). Meeting report: Exploiting the tumor microenvironment for therapeutics. Cancer Research, 66, 4558–4560.
Sun, X., Kanwar, J. R., Leung, E., Lehnert, K., Wang, D., & Krissansen, G. W. (2001). Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Therapy, 8, 638–645.
Zhang, X., Kon, T., Wang, H., Li, F., Huang, Q., Rabbani, Z. N., et al. (2004). Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Research, 64, 8139–8142.
Chang, Q., Qin, R., Huang, T., Gao, J., & Feng, Y. (2006). Effect of antisense hypoxia-inducible factor 1alpha on progression, metastasis, and chemosensitivity of pancreatic cancer. Pancreas, 32, 297–305.
Li, L., Lin, X., Staver, M., Shoemaker, A., Semizarov, D., Fesik, S. W., et al. (2005). Evaluating hypoxia-inducible factor-1alpha as a cancer therapeutic target via inducible RNA interference in vivo. Cancer Research, 65, 7249–7258.
Dachs, G. U., Patterson, A. V., Firth, J. D., Ratcliffe, P. J., Townsend, K. M., Stratford, I. J., et al. (1997). Targeting gene expression to hypoxic tumor cells. Nature Medicine, 3, 515–520.
Cuevas, Y., Hernandez-Alcoceba, R., Aragones, J., Naranjo-Suarez, S., Castellanos, M. C., Esteban, M. A., et al. (2003). Specific oncolytic effect of a new hypoxia-inducible factor-dependent replicative adenovirus on von Hippel-Lindau-defective renal cell carcinomas. Cancer Research, 63, 6877–6884.
Brown, J. M. (1993). SR 4233 (tirapazamine): A new anticancer drug exploiting hypoxia in solid tumours. British Journal of Cancer, 67, 1163–1170.
Peters, K. B., & Brown, J. M. (2002). Tirapazamine: A hypoxia-activated topoisomerase II poison. Cancer Research, 62, 5248–5253.
von Pawel, J., von Roemeling, R., Gatzemeier, U., Boyer, M., Elisson, L. O., Clark, P., et al. (2000). Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: A report of the international CATAPULT I study group. Cisplatin and Tirapazamine in subjects with advanced previously untreated non-small-cell lung tumors. Journal of Clinical Oncology, 18, 1351–1359.
Williamson, S. K., Crowley, J. J., Lara, P. N., Jr., McCoy, J., Lau, D. H., Tucker, R. W., et al. (2005). Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. Journal of Clinical Oncology, 23, 9097–9104.
Rischin, D., Peters, L., Fisher, R., Macann, A., Denham, J., Poulsen, M., et al. (2005). Tirapazamine, Cisplatin, and Radiation versus Fluorouracil, Cisplatin, and radiation in patients with locally advanced head and neck cancer: A randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). Journal of Clinical Oncology, 23, 79–87.
Lemmon, M. J., van Zijl, P., Fox, M. E., Mauchline, M. L., Giaccia, A. J., Minton, N. P., et al. (1997). Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Therapy, 4, 791–796.
Liu, S. C., Minton, N. P., Giaccia, A. J., & Brown, J. M. (2002). Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Therapy, 9, 291–296.
Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W., & Vogelstein, B. (2001). Combination bacteriolytic therapy for the treatment of experimental tumors. Proceedings of the National Academy of Sciences of the United States of America, 98, 15155–15160.
Cheong, I., Huang, X., Bettegowda, C., Diaz, L. A., Jr., Kinzler, K. W., Zhou, S., et al. (2006). A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science, 314, 1308–1311.
Schioppa, T., Uranchimeg, B., Saccani, A., Biswas, S. K., Doni, A., Rapisarda, A., et al. (2003). Regulation of the chemokine receptor CXCR4 by hypoxia. Journal of Experimental Medicine, 198, 1391–1402.
Pennacchietti, S., Michieli, P., Galluzzo, M., Mazzone, M., Giordano, S., & Comoglio, P. M. (2003). Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell, 3, 347–361.
Erler, J. T., Bennewith, K. L., Nicolau, M., Dornhofer, N., Kong, C., Le, Q. T., et al. (2006). Lysyl oxidase is essential for hypoxia-induced metastasis. Nature, 440, 1222–1226.
Ferrara, N. (2005). VEGF as a therapeutic target in cancer. Oncology, 69(Suppl 3), 11–16.
Park, E. J., Kong, D., Fisher, R., Cardellina, J., Shoemaker, R. H., & Melillo, G. (2006). Targeting the PAS-a domain of HIF-1alpha for development of small molecule inhibitors of HIF-1. Cell Cycle, 5(16), 1847–1853.
Zundel, W., Schindler, C., Haas-Kogan, D., Koong, A., Kaper, F., Chen, E., et al. (2000). Loss of PTEN facilitates HIF-1-mediated gene expression. Genes & Development, 14, 391–396.
Majumder, P. K., Febbo, P. G., Bikoff, R., Berger, R., Xue, Q., McMahon, L. M., et al. (2004). mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nature Medicine, 10, 594–601.
Thomas, G. V., Tran, C., Mellinghoff, I. K., Welsbie, D. S., Chan, E., Fueger, B., et al. (2006). Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nature Medicine, 12, 2–127.
Del Bufalo, D., Ciuffreda, L., Trisciuoglio, D., Desideri, M., Cognetti, F., Zupi, G., et al. (2006). Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Research, 66, 5549–5554.
Calvani, M., Rapisarda, A., Uranchimeg, B., Shoemaker, R. H., & Melillo, G. (2005). Hypoxic induction of a HIF-1{alpha}-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood, 107, 2705–2712.
Wan, X., Shen, N., Mendoza, A., Khanna, C., & Helman, L. J. (2006). CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia, 8, 394–401.
Zhong, H., Chiles, K., Feldser, D., Laughner, E., Hanrahan, C., Georgescu, M. M., et al. (2000). Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Research, 60, 1541–1545.
Pore, N., Jiang, Z., Gupta, A., Cerniglia, G., Kao, G. D., & Maity, A. (2006). EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Research, 66, 3197–3204.
Peng, X., Karna, P., Cao, Z., Jiang, B., Zhou, M., & Yang, L. (2006). Cross-talk between epidermal growth factor receptor and HIF-1 signal pathways increases resistance to apoptosis by upregulating survivin gene expression. Journal of Biological Chemistry 281, 25903–25914.
Luwor, R. B., Lu, Y., Li, X., Mendelsohn, J., & Fan, Z. (2005). The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene, 24, 4433–4441.
Laughner, E., Taghavi, P., Chiles, K., Mahon, P. C., & Semenza, G. L. (2001). HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Molecular and Cellular Biology, 21, 3995–4004.
Koukourakis, M. I., Simopoulos, C., Polychronidis, A., Perente, S., Botaitis, S., Giatromanolaki, A., et al. (2003). The effect of trastuzumab/docatexel combination on breast cancer angiogenesis: Dichotomus effect predictable by the HIFI alpha/VEGF pre-treatment status? Anticancer Research, 23, 1673–1680.
Mayerhofer, M., Valent, P., Sperr, W. R., Griffin, J. D., & Sillaber, C. (2002). BCR/ABL induces expression of vascular endothelial growth factor and its transcriptional activator, hypoxia inducible factor-1alpha, through a pathway involving phosphoinositide 3-kinase and the mammalian target of rapamycin. Blood, 100, 3767–3775.
Litz, J., & Krystal, G. W. (2006). Imatinib inhibits c-Kit-induced hypoxia-inducible factor-1alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Molecular Cancer Therapy, 5, 1415–1422.
Cao, Z., Fang, J., Xia, C., Shi, X., & Jiang, B. H. (2004). trans-3,4,5′-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clinical Cancer Research, 10, 5253–5263.
Fang, J., Cao, Z., Chen, Y. C., Reed, E., & Jiang, B. H. (2004). 9-beta-D-arabinofuranosyl-2-fluoroadenine inhibits expression of vascular endothelial growth factor through hypoxia-inducible factor-1 in human ovarian cancer cells. Molecular Pharmacology, 66, 178–186.
Fang, J., Xia, C., Cao, Z., Zheng, J. Z., Reed, E., & Jiang, B. H. (2005). Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB Journal, 19, 342–353.
Pore, N., Gupta, A. K., Cerniglia, G. J., Jiang, Z., Bernhard, E. J., Evans, S. et al. (2006). Nelfinavir down-regulates hypoxia-inducible factor 1{alpha} and VEGF expression and increases tumor oxygenation: Implications for radiotherapy. Cancer Research, 66, 9252–9259.
Tan, C., de Noronha, R. G., Roecker, A. J., Pyrzynska, B., Khwaja, F., Zhang, Z., et al. (2005). Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1 pathway. Cancer Research, 65, 605–612.
Zhang, Q., Tang, X., Lu, Q., Zhang, Z., Rao, J., & Le, A. D. (2006). Green tea extract and (−)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Molecular Cancer Therapy, 5, 1227–1238.
Zhang, Q., Tang, X., Lu, Q. Y., Zhang, Z. F., Brown, J., & Le, A. D. (2005). Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Molecular Cancer Therapy, 4, 1465–1474.
Zhong, X. S., Zheng, J. Z., Reed, E., & Jiang, B. H. (2004). SU5416 inhibited VEGF and HIF-1alpha expression through the PI3K/AKT/p70S6K1 signaling pathway. Biochemical and Biophysical Research Communications, 324, 471–480.
Pommier, Y. (2006). Topoisomerase I inhibitors: Camptothecins and beyond. Nature Reviews Cancer, 6, 789–802.
Rapisarda, A., Uranchimeg, B., Scudiero, D. A., Selby, M., Sausville, E. A., Shoemaker, R. H., et al. (2002). Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Research, 62, 4316–4324.
Rapisarda, A., Uranchimeg, B., Sordet, O., Pommier, Y., Shoemaker, R. H., & Melillo, G. (2004). Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: Mechanism and therapeutic implications. Cancer Research, 64, 1475–1482.
Rapisarda, A., Zalek, J., Hollingshead, M., Braunschweig, T., Uranchimeg, B., Bonomi, C. A., et al. (2004). Schedule-dependent inhibition of hypoxia-inducible factor-1alpha protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Research, 64, 6845–6848.
Mabjeesh, N. J., Escuin, D., LaVallee, T. M., Pribluda, V. S., Swartz, G. M., Johnson, M. S. P., et al. (2003). 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell, 3, 363–375.
Escuin, D., Kline, E. R., & Giannakakou, P. (2005). Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Research, 65, 9021–9028.
Jung, Y. J., Isaacs, J. S., Lee, S., Trepel, J., & Neckers, L. (2003). Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. Journal of Biological Chemistry, 278, 7445–7452.
Kang, S. H., Cho, H. T., Devi, S., Zhang, Z., Escuin, D., Liang, Z., et al. (2006). Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Research, 66, 11991–11997.
Ricker, J. L., Chen, Z., Yang, X. P., Pribluda, V. S., Swartz, G. M., & Van Waes, C. (2004). 2-methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clinical Cancer Research, 10, 8665–8673.
Isaacs, J. S., Jung, Y. J., Mimnaugh, E. G., Martinez, A., Cuttitta, F., & Neckers, L. M. (2002). Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. Journal of Biological Chemistry, 277, 29936–29944.
Mabjeesh, N. J., Post, D. E., Willard, M. T., Kaur, B., Van Meir, E. G., Simons, J. W., et al. (2002). Geldanamycin induces degradation of hypoxia-inducible factor 1alpha protein via the proteosome pathway in prostate cancer cells. Cancer Research, 62, 2478–2482.
Hur, E., Kim, H. H., Choi, S. M., Kim, J. H., Yim, S., Kwon, H. J., et al. (2002). Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Molecular Pharmacology, 62, 975–982.
Kurebayashi, J., Otsuki, T., Kurosumi, M., Soga, S., Akinaga, S., & Sonoo, H. (2001). A radicicol derivative, KF58333, inhibits expression of hypoxia-inducible factor-1alpha and vascular endothelial growth factor, angiogenesis and growth of human breast cancer xenografts. Japanese Journal of Cancer Research, 92, 1342–1351.
Han, J. Y., Oh, S. H., Morgillo, F., Myers, J. N., Kim, E., Hong, W. K., et al. (2005). Hypoxia-inducible factor 1alpha and antiangiogenic activity of farnesyltransferase inhibitor SCH66336 in human aerodigestive tract cancer. Journal of the National Cancer Institute, 97, 1272–1286.
Osada, M., Imaoka, S., & Funae, Y. (2004). Apigenin suppresses the expression of VEGF, an important factor for angiogenesis, in endothelial cells via degradation of HIF-1alpha protein. FEBS Letters, 575, 59–63.
Kim, M. S., Kwon, H. J., Lee, Y. M., Baek, J. H., Jang, J. E., Lee, S. W., et al. (2001). Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nature Medicine, 7, 437–443.
Fath, D. M., Kong, X., Liang, D., Lin, Z., Chou, A., Jiang, Y., et al. (2006). Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-alpha. Journal of Biological Chemistry, 281, 13612–13619.
Kong, X., Lin, Z., Liang, D., Fath, D., Sang, N., & Caro, J. (2006). Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Molecular and Cellular Biology, 26, 2019–2028.
Qian, D. Z., Kachhap, S. K., Collis, S. J., Verheul, H. M. W., Carducci, M. A., Atadja, P., et al. (2006). Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1{alpha}. Cancer Research, 66, 8814–8821.
Chun, Y. S., Yeo, E. J., & Park, J. W. (2004). Versatile pharmacological actions of YC-1: Anti-platelet to anticancer. Cancer Letters, 207, 1–7.
Chun, Y. S., Yeo, E. J., Choi, E., Teng, C. M., Bae, J. M., Kim, M. S., et al. (2001). Inhibitory effect of YC-1 on the hypoxic induction of erythropoietin and vascular endothelial growth factor in Hep3B cells. Biochemical Pharmacology, 61, 947–954.
Yeo, E. J., Chun, Y. S., Cho, Y. S., Kim, J., Lee, J. C., Kim, M. S., et al. (2003). YC-1: A potential anticancer drug targeting hypoxia-inducible factor 1. Journal of the National Cancer Institute, 95, 516–525.
Kim, H. L., Yeo, E. J., Chun, Y. S., & Park, J. W. (2006). A domain responsible for HIF-1alpha degradation by YC-1, a novel anticancer agent. International Journal of Oncology, 29, 255–260.
Welsh, S., Williams, R., Kirkpatrick, L., Paine-Murrieta, G., & Powis, G. (2004). Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Molecular Cancer Therapy, 3, 233–244.
Welsh, S. J., Bellamy, W. T., Briehl, M. M., & Powis, G. (2002). The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Research, 62, 5089–5095.
Welsh, S. J., Williams, R. R., Birmingham, A., Newman, D. J., Kirkpatrick, D. L., & Powis, G. (2003). The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Molecular Cancer Therapy, 2, 235–243.
Jones, D. T., & Harris, A. L. (2006). Identification of novel small-molecule inhibitors of hypoxia-inducible factor-1 transactivation and DNA binding. Molecular Cancer Therapy, 5, 2193–2202.
Chau, N. M., Rogers, P., Aherne, W., Carroll, V., Collins, I., McDonald, E., et al. (2005). Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors. Cancer Research, 65, 4918–4928.
Jones, M. K., Szabo, I. L., Kawanaka, H., Husain, S. S., & Tarnawski, A. S. (2002). von Hippel Lindau tumor suppressor and HIF-1alpha: New targets of NSAIDs inhibition of hypoxia-induced angiogenesis. FASEB Journal, 16, 264–266.
Palayoor, S. T., Tofilon, P. J., & Coleman, C. N. (2003). Ibuprofen-mediated reduction of hypoxia-inducible factors HIF-1alpha and HIF-2alpha in prostate cancer cells. Clinical Cancer Research, 9, 3150–3157.
Zhong, H., Willard, M., & Simons, J. (2004). NS398 reduces hypoxia-inducible factor (HIF)-1alpha and HIF-1 activity: Multiple-level effects involving cyclooxygenase-2 dependent and independent mechanisms. International Journal of Cancer, 112, 585–595.
Knowles, H. J., Raval, R. R., Harris, A. L., & Ratcliffe, P. J. (2003). Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Research, 63, 1764–1768.
Melillo, G., Sausville, E. A., Cloud, K., Lahusen, T., Varesio, L., & Senderowicz, A. M. (1999). Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Research, 59, 5433–5437.
Newcomb, E. W., Ali, M. A., Schnee, T., Lan, L., Lukyanov, Y., Fowkes, M., et al. (2005). Flavopiridol downregulates hypoxia-mediated hypoxia-inducible factor-1alpha expression in human glioma cells by a proteasome-independent pathway: Implications for in vivo therapy. Journal of Neuro-Oncology, 7, 225–235.
Buchler, P., Reber, H. A., Buchler, M. W., Friess, H., Lavey, R. S., & Hines, O. J. (2004). Antiangiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer, 100, 201–210.
Dervan, P. B., & Edelson, B. S. (2003). Recognition of the DNA minor groove by pyrrole-imidazole polyamides. Current Opinion in Structural Biology, 13, 284–299.
Olenyuk, B. Z., Zhang, G. J., Klco, J. M., Nickols, N. G., Kaelin, W. G., Jr., & Dervan, P. B. (2004). Inhibition of vascular endothelial growth factor with a sequence-specific hypoxia response element antagonist. Proceedings of the National Academy of Sciences of the United States of America, 101, 16768–16773.
Nickols, N. G., Jacobs, C. S., Farkas, M. E., & Dervan, P. B. (2007). Improved nuclear localization of DNA-binding polyamides. Nucleic Acids Research, 35(2), 363–370.
Van Dyke, M. M., & Dervan, P. B. (1984). Echinomycin binding sites on DNA. Science, 225, 1122–1127.
Kong, D., Park, E. J., Stephen, A. G., Calvani, M., Cardellina, J. H., Monks, A., et al. (2005). Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Research, 65, 9047–9055.
Kung, A. L., Wang, S., Klco, J. M., Kaelin, W. G., & Livingston, D. M. (2000). Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nature Medicine, 6, 1335–1340.
Kung, A. L., Zabludoff, S. D., France, D. S., Freedman, S. J., Tanner, E. A., Vieira, A., et al. (2004). Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell, 6, 33–43.
Kaluz, S., Kaluzova, M., & Stanbridge, E. J. (2006). Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1alpha C-terminal activation domain. Molecular and Cellular Biology, 26, 5895–5907.
Yeo, E. J., Ryu, J. H., Cho, Y. S., Chun, Y. S., Huang, L. E., Kim, M. S., et al. (2006). Amphotericin B blunts erythropoietin response to hypoxia by reinforcing FIH-mediated repression of HIF-1. Blood, 107, 916–923.
Nagle, D. G., & Zhou, Y. D. (2006). Natural product-based inhibitors of hypoxia-inducible factor-1 (HIF-1). Current Drugs Targets, 7, 355–369.
Dai, J., Fishback, J. A., Zhou, Y. D., & Nagle, D. G. (2006). Sodwanone and Yardenone Triterpenes from a South African species of the marine sponge Axinella inhibit Hypoxia-Inducible Factor-1 (HIF-1) activation in both breast and prostate tumor cells. Journal of Natural Products, 69, 1715–1720.
Hodges, T. W., Hossain, C. F., Kim, Y. P., Zhou, Y. D., & Nagle, D. G. (2004). Molecular-targeted antitumor agents: The Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. Journal of Natural Products, 67, 767–771.
Mohammed, K. A., Hossain, C. F., Zhang, L., Bruick, R. K., Zhou, Y. D., & Nagle, D. G. (2004). Laurenditerpenol, a new diterpene from the tropical marine alga Laurenciaintricata that potently inhibits HIF-1 mediated hypoxic signaling in breast tumor cells. Journal of Natural Products, 67, 2002–2007.
Zhou, Y. D., Kim, Y. P., Mohammed, K. A., Jones, D. K., Muhammad, I., Dunbar, D. C., et al. (2005). Terpenoid tetrahydroisoquinoline alkaloids emetine, klugine, and isocephaeline inhibit the activation of hypoxia-inducible factor-1 in breast tumor cells. Journal of Natural Products, 68, 947–950.
Choi, H., Chun, Y. S., Kim, S. W., Kim, M. S., & Park, J. W. (2006). Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: A mechanism of tumor growth inhibition. Molecular Pharmacology, 70, 1664–1671.
Lin, S., Tsai, S. C., Lee, C. C., Wang, B. W., Liou, J. Y., & Shyu, K. G. (2004). Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Molecular Pharmacology, 66, 612–619.
Li, M. H., Miao, Z. H., Tan, W. F., Yue, J. M., Zhang, C., Lin, L. P., et al. (2004). Pseudolaric acid B inhibits angiogenesis and reduces hypoxia-inducible factor 1alpha by promoting proteasome-mediated degradation. Clinical Cancer Research, 10, 8266–8274.
Cai, X. F., Jin, X., Lee, D., Yang, Y. T., Lee, K., Hong, Y. S., et al. (2006). Phenanthroquinolizidine alkaloids from the roots of Boehmeria pannosa potently inhibit Hypoxia-Inducible Factor-1 in AGS human gastric cancer cells. Journal of Natural Products, 69, 1095–1097.
Hasebe, Y., Egawa, K., Yamazaki, Y., Kunimoto, S., Hirai, Y., Ida, Y., et al. (2003). Specific inhibition of hypoxia-Inducible Factor (HIF)-1 alpha activation and of vascular endothelial growth factor (VEGF) production by flavonoids. Biological & Pharmaceutical Bulletin, 26, 1379–1383.
Li, L., Lin, X., Shoemaker, A. R., Albert, D. H., Fesik, S. W., & Shen, Y. (2006). Hypoxia-Inducible Factor-1 inhibition in combination with temozolomide treatment exhibits robust antitumor efficacy in vivo. Clinical Cancer Research, 12, 4747–4754.
Brown, L. M., Cowen, R. L., Debray, C., Eustace, A., Erler, J. T., Sheppard, F. C., et al. (2005). Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting Hypoxia Inducible Factor. Molecular Pharmacology, 69, 411–418.
Moeller, B. J., Cao, Y., Li, C. Y., & Dewhirst, M. W. (2004). Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell, 5, 429–441.
Moeller, B. J., Dreher, M. R., Rabbani, Z. N., Schroeder, T., Cao, Y., Li, C. Y., et al. (2005). Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell, 8, 99–110.
Mie, L. Y., Kim, S. H., Kim, H. S., Jin, S. M., Nakajima, H., Jeong, K. H., et al. (2003). Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochemical and Biophysical Research Communications, 300, 241–246.
Jones, D. T., Pugh, C. W., Wigfield, S., Stevens, M. F. G., & Harris, A. L. (2006). Novel thioredoxin inhibitors paradoxically increase Hypoxia-Inducible Factor-{alpha} expression but decrease functional transcriptional activity, DNA binding, and degradation. Clinical Cancer Research, 12, 5384–5394.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melillo, G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev 26, 341–352 (2007). https://doi.org/10.1007/s10555-007-9059-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-007-9059-x