Abstract
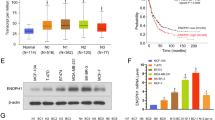
Enolase-α (ENO-1) is a key glycolytic enzyme that has been used as a diagnostic marker to identify human lung cancers. To investigate the role of ENO-1 in breast cancer diagnosis and therapy, the mRNA levels of ENO-1 in 244 tumor and normal paired tissue samples and 20 laser capture-microdissected cell clusters were examined by quantitative real-time PCR analysis. Increased ENO-1 mRNA expression was preferentially detected in estrogen receptor-positive (ER+) tumors (tumor/normal ratio >90-fold) when compared to ER-negative (tumor/normal ratio >20-fold) tumor tissues. The data presented here demonstrate that those patients whose tumors highly expressed ENO-1 had a poor prognosis with greater tumor size (>2 cm, *P = .017), poor nodal status (N > 3, *P = .018), and a shorter disease-free interval (≦1 year, *P < .009). We also found that higher-expressing ENO-1 tumors confer longer distance relapse (tumor/normal ratio = 82.8–92.4-fold) when compared to locoregional relapse (tumor/normal ratio = 43.4-fold) in postsurgical 4-hydroxy-tamoxifen (4-OHT)-treated ER+ patients (*P = .014). These data imply that changes in tumor ENO-1 levels are related to clinical 4-OHT therapeutic outcome. In vitro studies demonstrated that decreasing ENO-1 expression using small interfering RNA (siRNA) significantly augmented 4-OHT (100 nM)-induced cytotoxicity in tamoxifen-resistant (Tam-R) breast cancer cells. These results suggest that downregulation of ENO-1 could be utilized as a novel pharmacological approach for overcoming 4-OHT resistance in breast cancer therapy.






Similar content being viewed by others
Abbreviations
- ChIP:
-
Chromatin immunoprecipitation
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethylsulfoxide
- E:
-
Estrogens
- DOX:
-
Doxorubicin
- ENO-1:
-
Enolase α
- ENO-2:
-
Enolase β
- ENO-3:
-
Enolase γ
- ER:
-
Estrogen receptor
- FACS:
-
Fluorescence-activated cell sorter
- FAS:
-
Fetal calf serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GF:
-
Griseofulvin
- GUS:
-
β-Glucuronidase
- IHC:
-
Immunohistochemistry
- LCM:
-
Laser capture microdissection
- MBP-1:
-
c-Myc promoter-binding protein
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
- NFκB:
-
Nuclear factor kappa B
- 4-OHT:
-
4-Hydroxytamoxifen
- PBS:
-
Phosphate-buffered saline
- PDTC:
-
Pyrrolidine dithiocarbamate
- PR:
-
Progesterone receptor
- PTX:
-
Paclitaxel
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- siRNA:
-
Small interfering RNA
- TBP:
-
TATA-box-binding protein
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Dodwell D, Williamson D (2008) Beyond tamoxifen: extended and late extended endocrine therapy in postmenopausal early breast cancer. Cancer Treat Rev 34:137–144
Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN (1995) Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol 13:513–529
Chen B, Wang Y, Kane SE, Chen S (2008) Improvement of sensitivity to tamoxifen in estrogen receptor-positive and Herceptin-resistant breast cancer cells. J Mol Endocrinol 41:367–377
Kim SK, Yang JW, Kim MR, Roh SH, Kim HG, Lee KY, Jeong HG, Kang KW (2008) Increased expression of Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant breast cancer cells. Free Radic Biol Med 45:537–546
Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S (2008) Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res 68:4910–4918
Honorat M, Mesnier A, Vendrell J, Guitton J, Bieche I, Lidereau R, Kruh GD, Dumontet C, Cohen P, Payen L (2008) ABCC11 expression is regulated by estrogen in MCF7 cells, correlated with estrogen receptor alpha expression in postmenopausal breast tumors and overexpressed in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer 15:125–138
Lebioda L, Stec B (1988) Crystal structure of enolase indicates that enolase and pyruvate kinase evolved from a common ancestor. Nature 333:683–686
Chi-Shing Cho W (2007) Potentially useful biomarkers for the diagnosis, treatment and prognosis of lung cancer. Biomed Pharmacother 61:515–519
Horiatis D, Wang Q, Pinski J (2004) A new screening system for proliferation-independent anti-cancer agents. Cancer Lett 210:119–124
Riby JE, Firestone GL, Bjeldanes LF (2008) 3, 3′-diindolylmethane reduces levels of HIF-1alpha and HIF-1 activity in hypoxic cultured human cancer cells. Biochem Pharmacol 75:1858–1867
Sims PA, Menefee AL, Larsen TM, Mansoorabadi SO, Reed GH (2006) Structure and catalytic properties of an engineered heterodimer of enolase composed of one active and one inactive subunit. J Mol Biol 355:422–431
Chang YS, Wu W, Walsh G, Hong WK, Mao L (2003) Enolase-alpha is frequently down-regulated in non-small cell lung cancer and predicts aggressive biological behavior. Clin Cancer Res 9:3641–3644
Ray R, Miller DM (1991) Cloning and characterization of a human c-myc promoter-binding protein. Mol Cell Biol 11:2154–2161
Subramanian A, Miller DM (2000) Structural analysis of alpha-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene. J Biol Chem 275:5958–5965
Fan P, Wang J, Santen RJ, Yue W (2007) Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 67:1352–1360
Aerts JL, Gonzales MI, Topalian SL (2004) Selection of appropriate control genes to assess expression of tumor antigens using real-time RT-PCR. Biotechniques 36:84–86, 88, 90–91
Chen F, Kim E, Wang CC, Harrison LE (2005) Ciglitazone-induced p27 gene transcriptional activity is mediated through Sp1 and is negatively regulated by the MAPK signaling pathway. Cellular signalling 17:1572–1577
Chen CS, Wu CH, Lai YC, Lee WS, Chen HM, Chen RJ, Chen LC, Ho YS, Wang YJ (2008) NF-kappaB-activated tissue transglutaminase is involved in ethanol-induced hepatic injury and the possible role of propolis in preventing fibrogenesis. Toxicology 246:148–157
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14:518–527
Wilkinson DS, Ogden SK, Stratton SA, Piechan JL, Nguyen TT, Smulian GA, Barton MC (2005) A direct intersection between p53 and transforming growth factor beta pathways targets chromatin modification and transcription repression of the alpha-fetoprotein gene. Molecular and cellular biology 25:1200–1212
Tiseo M, Ardizzoni A, Cafferata MA, Loprevite M, Chiaramondia M, Filiberti R, Marroni P, Grossi F, Paganuzzi M (2008) Predictive and prognostic significance of neuron-specific enolase (NSE) in non-small cell lung cancer. Anticancer Res 28:507–513
He P, Naka T, Serada S, Fujimoto M, Tanaka T, Hashimoto S, Shima Y, Yamadori T, Suzuki H, Hirashima T, Matsui K, Shiono H, Okumura M, Nishida T, Tachibana I, Norioka N, Norioka S, Kawase I (2007) Proteomics-based identification of alpha-enolase as a tumor antigen in non-small lung cancer. Cancer Sci 98:1234–1240
Chang GC, Liu KJ, Hsieh CL, Hu TS, Charoenfuprasert S, Liu HK, Luh KT, Hsu LH, Wu CW, Ting CC, Chen CY, Chen KC, Yang TY, Chou TY, Wang WH, Whang-Peng J, Shih NY (2006) Identification of alpha-enolase as an autoantigen in lung cancer: its overexpression is associated with clinical outcomes. Clin Cancer Res 12:5746–5754
Panasci L, Jean-Claude BJ, Vasilescu D, Mustafa A, Damian S, Damian Z, Georges E, Liu Z, Batist G, Leyland-Jones B (1996) Sensitization to doxorubicin resistance in breast cancer cell lines by tamoxifen and megestrol acetate. Biochem Pharmacol 52:1097–1102
Lavie Y, Cao H, Volner A, Lucci A, Han TY, Geffen V, Giuliano AE, Cabot MC (1997) Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem 272:1682–1687
Fujita T, Washio K, Takabatake D, Takahashi H, Yoshitomi S, Tsukuda K, Ishibe Y, Ogasawara Y, Doihara H, Shimizu N (2005) Proteasome inhibitors can alter the signaling pathways and attenuate the P-glycoprotein-mediated multidrug resistance. Int J Cancer 117:670–682
Ho YS, Duh JS, Jeng JH, Wang YJ, Liang YC, Lin CH, Tseng CJ, Yu CF, Chen RJ, Lin JK (2001) Griseofulvin potentiates antitumorigenesis effects of nocodazole through induction of apoptosis and G2/M cell cycle arrest in human colorectal cancer cells. Int J Cancer 91:393–401
Hermeking H, Eick D (1994) Mediation of c-Myc-induced apoptosis by p53. Science 265:2091–2093
Ray RB, Steele R, Seftor E, Hendrix M (1995) Human breast carcinoma cells transfected with the gene encoding a c-myc promoter-binding protein (MBP-1) inhibits tumors in nude mice. Cancer Res 55:3747–3751
Kang Y, Cortina R, Perry RR (1996) Role of c-myc in tamoxifen-induced apoptosis estrogen-independent breast cancer cells. J Natl Cancer Inst 88:279–284
Mandlekar S, Kong AN (2001) Mechanisms of tamoxifen-induced apoptosis. Apoptosis 6:469–477
Planas-Silva MD, Hamilton KN (2007) Targeting c-Src kinase enhances tamoxifen’s inhibitory effect on cell growth by modulating expression of cell cycle and survival proteins. Cancer Chemother Pharmacol 60:535–543
Zheng A, Kallio A, Harkonen P (2007) Tamoxifen-induced rapid death of MCF-7 breast cancer cells is mediated via extracellularly signal-regulated kinase signaling and can be abrogated by estrogen. Endocrinology 148:2764–2777
Webb P, Lopez GN, Uht RM, Kushner PJ (1995) Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456
Vendrell JA, Bieche I, Desmetz C, Badia E, Tozlu S, Nguyen C, Nicolas JC, Lidereau R, Cohen PA (2005) Molecular changes associated with the agonist activity of hydroxy-tamoxifen and the hyper-response to estradiol in hydroxy-tamoxifen-resistant breast cancer cell lines. Endocr Relat Cancer 12:75–92
Quaedackers ME, van den Brink CE, van der Saag PT, Tertoolen LG (2007) Direct interaction between estrogen receptor alpha and NF-kappaB in the nucleus of living cells. Mol Cell Endocrinol 273:42–50
Kalaitzidis D, Gilmore TD (2005) Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab 16:46–52
Zhou Y, Eppenberger-Castori S, Marx C, Yau C, Scott GK, Eppenberger U, Benz CC (2005) Activation of nuclear factor-kappaB (NFkappaB) identifies a high-risk subset of hormone-dependent breast cancers. Int J Biochem Cell Biol 37:1130–1144
Harvell DM, Richer JK, Singh M, Spoelstra N, Finlayson C, Borges VF, Elias AD, Horwitz KB (2008) Estrogen regulated gene expression in response to neoadjuvant endocrine therapy of breast cancers: tamoxifen agonist effects dominate in the presence of an aromatase inhibitor. Breast Cancer Res Treat 112:489–501
Ali S, Coombes RC (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2:101–112
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712
Elledge RM, Green S, Ciocca D, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, O’Sullivan J, Martino S, Osborne CK (1998) HER-2 expression and response to tamoxifen in estrogen receptor-positive breast cancer: a southwest oncology group study. Clin Cancer Res 4:7–12
John S, Nayvelt I, Hsu HC, Yang P, Liu W, Das GM, Thomas T, Thomas TJ (2008) Regulation of estrogenic effects by beclin 1 in breast cancer cells. Cancer Res 68:7855–7863
Peng QP, Zhou JM, Zhou Q, Pan F, Zhong DP, Liang HJ (2008) Downregulation of the hexokinase II gene sensitizes human colon cancer cells to 5-fluorouracil. Chemotherapy 54:357–363
Scatena R, Bottoni P, Pontoglio A, Mastrototaro L, Giardina B (2008) Glycolytic enzyme inhibitors in cancer treatment. Expert Opin Investig Drugs 17:1533–1545
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452:230–233
Malorni L, Cacace G, Cuccurullo M, Pocsfalvi G, Chambery A, Farina A, Di Maro A, Parente A, Malorni A (2006) Proteomic analysis of MCF-7 breast cancer cell line exposed to mitogenic concentration of 17beta-estradiol. Proteomics 6:5973–5982
Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES (2005) Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics 4:1686–1696
Ejma M, Misiuk-Hojlo M, Gorczyca WA, Podemski R, Szymaniec S, Kuropatwa M, Rogozinska-Szczepka J, Bartnik W (2008) Antibodies to 46-kDa retinal antigen in a patient with breast carcinoma and cancer-associated retinopathy. Breast Cancer Res Treat 110:269–271
Acknowledgments
This study was supported by the National Science Council, grant NSC 95-2320-B-038-016-MY3 for Dr. Ho, and NSC 96-2314-B-038-002 for Dr. Wu, and by the Cathay Medical Center (95CGH-TMU-09 and 96CGH-TMU-05).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2009_492_MOESM1_ESM.tif
Fig. S1 RT-PCR analyses for ENO-1 in human breast carcinoma tissues. mRNA expression levels for ENO-1 and GUS were detected as specific single bands (403 and 165 bp, respectively) in both tumor and adjacent normal tissues. PCR was performed for 35 cycles. One hundred cases are represented in this agarose gel image (TIFF 3,564 kb)
Rights and permissions
About this article
Cite this article
Tu, SH., Chang, CC., Chen, CS. et al. Increased expression of enolase α in human breast cancer confers tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat 121, 539–553 (2010). https://doi.org/10.1007/s10549-009-0492-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0492-0