Abstract
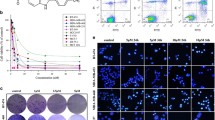
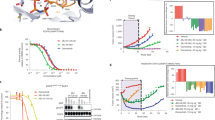
AKT and MAPK signaling are involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. RAS proteins are upstream mediators that transfer messages from surface receptors to intracellular signal transducers including MAPK and AKT pathways. AZD3409 is a novel prenyl inhibitor that has shown activity against both farnesyl transferase and geranylgeranyl transferase in isolated enzyme studies. We explored the activity of AZD3409 on breast cancer cell lines with high (SK-Br-3), intermediate (MDA-MB-361) or low (MDA-MB-468) sensitivity to gefitinib. We found that AZD3409 inhibits the growth of breast cancer cells in a dose-dependent manner, with the MDA-MB-468 and MDA-MB-361 cell lines showing higher sensitivity as compared with SK-Br-3 cells. Treatment with AZD3409 produced a significant reduction in the levels of activation of AKT in the three cell lines. AZD3409 also induced an increase in the expression of p27kip-1 and of hypophosphorylated forms of pRb2 in MDA-MB-468 cells that was associated with accumulation of cells in G0/G1 and the appearance of a sub-G1 peak suggestive of apoptosis. In contrast, AZD3409 produced a G2 arrest associated with reduced expression of pRb2 in MDA-MB-361 cells. A synergistic anti-tumor effect was observed when MDA-MB-468 or MDA-MB-361 cells were treated with both AZD3409 and gefitinib, whereas this combination was only additive in SK-Br-3 cells. However, treatment of breast cancer cells with AZD3409 and gefitinib did not produce a more significant blockade of AKT signaling as compared with gefitinib alone. These data suggest that AZD3409 might be active in gefitinib-resistant breast carcinoma.
Similar content being viewed by others
References
Agrawal A, Gutteridge E, Gee JM, Nicholson RI, Robertson JF (2005) Overview of tyrosine kinase inhibitors in clinical breast cancer. Endocr Relat Cancer 12(Suppl 1):S135–S144
Anido J, Matar P, Albanell J, Guzman M, Rojo F, Arribas J, Averbuch S, Baselga J (2003) ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER2-overexpressing breast cancer cells. Clin Cancer Res 9:1274–1283
Appels NM, Beijnen JH, Schellens JH (2005) Development of farnesyl transferase inhibitors: a review. Oncologist 10:565–578
Ashar HR, James L, Gray K, Carr D, McGuirk M, Maxwell E, Black S, Armstrong L, Doll RJ, Taveras AG, Bishop WR, Kirschmeier P (2001) The farnesyl transferase inhibitor SCH 66336 induces a G(2) →M or G(1) pause in sensitive human tumor cell lines. Exp Cell Res 262:17–27
Barbacid M (1987) ras genes. Annu Rev Biochem 56:779–827
Bianco R, Shin I, Ritter CA, Yakes FM, Basso A, Rosen N, Tsurutani J, Dennis PA, Mills GB, Arteaga CL (2003) Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 22:2812–2822
Campiglio M, Locatelli A, Olgiati C, Normanno N, Somenzi G, Vigano L, Fumagalli M, Menard S, Gianni L (2004) Inhibition of proliferation and induction of apoptosis in breast cancer cells by the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor ZD1839 (‘Iressa’) is independent of EGFR expression level. J Cell Physiol 198:259–268
Caraglia M, D’Alessandro AM, Marra M, Giuberti G, Vitale G, Viscomi C, Colao A, Prete SD, Tagliaferri P, Tassone P, Budillon A, Venuta S, Abbruzzese A (2004) The farnesyl transferase inhibitor R115777 (Zarnestra) synergistically enhances growth inhibition and apoptosis induced on epidermoid cancer cells by Zoledronic acid (Zometa) and Pamidronate. Oncogene 23:6900–6913
Carloni V, Vizzutti F, Pantaleo P (2005) Farnesyltransferase inhibitor, ABT-100, is a potent liver cancer chemopreventive agent. Clin Cancer Res 11:4266–4274
Chou TC, Talalay P (1984) Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Clark GJ, Der CJ (1995) Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat 35:133–144
de Bono JS, Tolcher AW, Rowinsky EK (2003) Farnesyltransferase inhibitors and their potential in the treatment of breast carcinoma. Semin Oncol 30:79–92
Efuet ET, Keyomarsi K (2006) Farnesyl and geranylgeranyl transferase inhibitors induce G1 arrest by targeting the proteasome. Cancer Res 66:1040–1051
Jiang K, Coppola D, Crespo NC, Nicosia SV, Hamilton AD, Sebti SM, Cheng JQ (2000) The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol 20:139–148
Johnston SR, Hickish T, Ellis P, Houston S, Kelland L, Dowsett M, Salter J, Michiels B, Perez-Ruixo JJ, Palmer P, Howes A (2003) Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol 21:2492–2499
Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ (1992) Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA 89:6403–6407
Kelland LR, Smith V, Valenti M, Patterson L, Clarke PA, Detre S, End D, Howes AJ, Dowsett M, Workman P, Johnston SR (2001) Preclinical antitumor activity and pharmacodynamic studies with the farnesyl protein transferase inhibitor R115777 in human breast cancer. Clin Cancer Res 7:3544–3550
Khafagy R, Stephens T, Hart C, Ramani V, Brown M, Clarke N (2004) In vitro effects of the prenyl transferase inhibitor AZD3409 on prostate cancer epithelial cells. J Clin Oncol (Meeting Abstracts) 22:4744
Manne V, Lee FY, Bol DK, Gullo-Brown J, Fairchild CR, Lombardo LJ, Smykla RA, Vite GD, Wen ML, Yu C, Wong TW, Hunt JT (2004) Apoptotic and cytostatic farnesyltransferase inhibitors have distinct pharmacology and efficacy profiles in tumor models. Cancer Res 64:3974–3980
McCormack P, Macpherson M, Wilson D, Lindsay A, Parry T, Holt A, Hughes A (2004) Pharmacokinetic characterization of the novel oral prenyl transferase inhibitor AZD3409: the first analysis in healthy male volunteers. J Clin Oncol (Meeting Abstracts) 22:3172
McCormick F (1994) Activators and effectors of ras p21 proteins. Curr Opin Genet Dev 4:71–76
Moasser MM, Basso A, Averbuch SD, Rosen N (2001) The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res 61:7184–7188
Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, Caponigro F, Salomon DS (2005) The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets 6:243–257
Normanno N, De Luca A, Maiello MR, Mancino M, D’Antonio A, Macaluso M, Caponigro F, Giordano A (2005) Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in breast cancer: current status and future development. Front Biosci 10:2611–2617
Normanno N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, Perrone F (2005) Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocr Relat Cancer 12:721–747
Normanno N, De Luca A, Maiello MR, Campiglio M, Napolitano M, Mancino M, Carotenuto A, Viglietto G, Menard S (2006) The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol 207:420–427
O’Regan RM, Khuri FR (2004) Farnesyl transferase inhibitors: the next targeted therapies for breast cancer?. Endocr Relat Cancer 11:191–205
Pan J, Yeung SC (2005) Recent advances in understanding the antineoplastic mechanisms of farnesyltransferase inhibitors. Cancer Res 65:9109–9112
Sepp-Lorenzino L, Rosen N (1998) A farnesyl-protein transferase inhibitor induces p21 expression and G1 block in p53 wild type tumor cells. J Biol Chem 273:20243–20251
She QB, Solit D, Basso A, Moasser MM (2003) Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3’-kinase/Akt pathway signaling. Clin Cancer Res 9:4340–4346
Stephens TC, Wardleworth MJ, Matusiak ZS, Ashton SE, Hancox UJ, Bate M, Ferguson R, Boyle T (2003) AZD3409, a novel, oral, prenyl transferase inhibitor with promising preclinical antitumour activity. 970 pp
Sun J, Blaskovich MA, Knowles D, Qian Y, Ohkanda J, Bailey RD, Hamilton AD, Sebti SM (1999) Antitumor efficacy of a novel class of non-thiol-containing peptidomimetic inhibitors of farnesyltransferase and geranylgeranyltransferase I: combination therapy with the cytotoxic agents cisplatin, Taxol, and gemcitabine. Cancer Res 59:4919–4926
Venkatasubbarao K, Choudary A, Freeman JW (2005) Farnesyl transferase inhibitor (R115777)-induced inhibition of STAT3(Tyr705) phosphorylation in human pancreatic cancer cell lines require extracellular signal-regulated kinases. Cancer Res 65:2861–2871
Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK (1997) K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 272:14459–14464
Acknowledgements
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC) e Ministero della Salute to N. Normanno. A. De Luca was supported by a fellowship form AIRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maiello, M.R., D’Alessio, A., De Luca, A. et al. AZD3409 inhibits the growth of breast cancer cells with intrinsic resistance to the EGFR tyrosine kinase inhibitor gefitinib. Breast Cancer Res Treat 102, 275–282 (2007). https://doi.org/10.1007/s10549-006-9340-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9340-7