Abstract
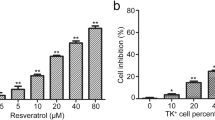
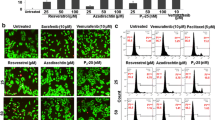
The incidence of melanoma continues to dramatically increase in most Western countries with predominantly Caucasian populations. However, only limited therapies for the metastatic stage of the disease are currently available. The main purpose of this study is to determine approaches that can substantially increase radiosensitivity of melanoma cells. The PI3K-AKT, NF-κB and COX-2 pathways, which are involved in the radioprotective response, are highly active in melanoma cells. Pharmacological suppression of COX-2 and PI3K-AKT, or RNAi-mediated knockdown of COX-2, substantially increased levels of G2/M arrest of the cell cycle and decreased clonogenic survival of gamma-irradiated melanomas, predominantly via a necrotic mechanism. On the other hand, resveratrol, a polyphenolic phytoalexin, selectively targets numerous cell signaling pathways, decreasing clonogenic survival primarily via an apoptotic mechanism. In melanoma cells, resveratrol inhibits STAT3 and NF-κB-dependent transcription, culminating in suppression of cFLIP and Bcl-xL expression, while activating the MAPK- and the ATM-Chk2-p53 pathways. Resveratrol also upregulates TRAIL promoter activity and induces TRAIL surface expression in some melanoma cell lines, resulting in a rapid development of apoptosis. Sequential treatment of melanoma cells, first with γ-irradiation to upregulate TRAIL-R surface expression, and then with resveratrol to suppress antiapoptotic proteins cFLIP and Bcl-xL and induce TRAIL surface expression, had dramatic effects on upregulation of apoptosis in some melanoma lines, including SW1 and WM35. However, for melanoma lines exhibiting suppressed translocation of TRAIL to the cell surface, a necrotic mechanism of cell death was primarily involved in radiation response. Hence, surface expression of TRAIL induced by resveratrol appears to be a decisive event, one which determines an apoptotic versus a necrotic response of melanoma cells to sequential treatment.







Similar content being viewed by others
References
Debatin KM, Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23:2950–2966
Okada H, Mak TW (2004) Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer 4:592–603
Cory S, Adams JM (2005) Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer Cell 8:5–6
Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436
Krammer PH, Kaminski M, Kiessling M, Gulow K (2007) No life without death. Adv Cancer Res 97C:111–138
Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Ashkenazi A (2008) Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ 15:751–761
Ashkenazi A (2002) Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer 2:420–430
Schaefer U, Voloshanenko O, Willen D, Walczak H (2007) TRAIL: a multifunctional cytokine. Front Biosci 12:3813–3824
Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL, Ross BD, Rehemtulla A (2000) Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA 97:1754–1759
Ivanov VN, Zhou H, Hei TK (2007) Sequential treatment by ionizing radiation and sodium arsenite dramatically accelerates TRAIL-mediated apoptosis of human melanoma cells. Cancer Res 67:5397–5407
Subbaramaiah K, Dannenberg AJ (2003) Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci 24:96–102
Luo J, Manning BD, Cantley LC (2003) Targeting the PI3K-AKT pathway in human cancer: rationale and promise. Cancer Cell 4:257–262
Satyamoorthy K, Chehab NH, Waterman MJ, Lien MC, El-Deiry WS, Herlyn M, Halazonetis TD (2000) Aberrant regulation and function of wild-type p53 in radioresistant melanoma cells. Cell Growth Differ 11:467–474
Vousden KH, Lane DP (2007) p53 in health and disease. Nat Rev Mol Cell Biol 8:275–283
Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P (2006) Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol 175:913–923
Gogvadze V, Orrenius S, Zhivotovsky B (2008) Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 18:165–173
Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK (2008) Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res 314:1163–1176
Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M (1997) Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res 7(Suppl 2):S35–S42
van Dam H, Huguier S, Kooistra K, Baguet J, Vial E, van der Eb AJ, Herrlich P, Angel P, Castellazzi M (1998) Autocrine growth and anchorage independence: two complementing Jun-controlled genetic programs of cellular transformation. Genes Dev 12:1227–1239
Baetu TM, Kwon H, Sharma S, Grandvaux N, Hiscott J (2001) Disruption of NF-kappaB signaling reveals a novel role for NF-kappaB in the regulation of TNF-related apoptosis-inducing ligand expression. J Immunol 167:3164–3173
Bartke T, Siegmund D, Peters N, Reichwein M, Henkler F, Scheurich P, Wajant H (2001) p53 upregulates cFLIP, inhibits transcription of NF-kappaB-regulated genes and induces caspase-8-independent cell death in DLD-1 cells. Oncogene 20:571–580
Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, El-Deiry WS (2004) Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol 24:8541–8555
Resnick-Silverman L, St Clair S, Maurer M, Zhao K, Manfredi JJ (1998) Identification of a novel class of genomic DNA-binding sites suggests a mechanism for selectivity in target gene activation by the tumor suppressor protein p53. Genes Dev 12:2102–2107
Bakhtiarova A, Taslimi P, Elliman SJ, Kosinski PA, Hubbard B, Kavana M, Kemp DM (2006) Resveratrol inhibits firefly luciferase. Biochem Biophys Res Commun 351:481–484
Ivanov VN, Hei TK (2004) Arsenite sensitizes human melanomas to apoptosis via tumor necrosis factor alpha-mediated pathway. J Biol Chem 279:22747–22758
Shiloh Y (2006) The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci 31:402–410
Nagata S (1999) Fas ligand-induced apoptosis. Annu Rev Genet 33:29–55
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Ivanov VN, Partridge MA, Johnson GE, Huang SXL, Zhou H, Hei TK (2008) Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res 314:1163–1176
Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res 24:2783–2840
Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R (2006) Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther 5:621–629
Rigel DS, Friedman RJ, Kopf AW (1996) The incidence of malignant melanoma in the United States: issues as we approach the 21st century. J Am Acad Derm 34:839–847
Rigel DS (2002) The effect of sunscreen on melanoma risk. Derm Clin 20:601–606
Perlis C, Herlyn M (2004) Recent advances in melanoma biology. Oncologist 9:182–187
Atkins MB (2006) Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res 12:2353s–2358s
Oehler C, Dickinson DJ, Broggini-Tenzer A, Hofstetter B, Hollenstein A, Riesterer O, Vuong V, Pruschy M (2007) Current concepts for the combined treatment modality of ionizing radiation with anticancer agents. Curr Pharm Des 13:519–535
Gwosdz C, Scheckenbach K, Lieven O, Reifenberger J, Knopf A, Bier H, Balz V (2006) Comprehensive analysis of the p53 status in mucosal and cutaneous melanomas. Int J Cancer 118:577–582
Hersey P, Zhang XD (2001) How melanoma cells evade trail-induced apoptosis. Nat Rev Cancer 1:142–150
Fulda S, Debatin KM (2004) Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res 64:337–346
Shankar S, Singh G, Srivastava RK (2007) Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci 12:4839–4854
Acknowledgments
We would like to thanks Drs. Z. Ronai, M. Herlyn, S. Y. Fuchs, H. B. Liberman and Y. Yin for discussion of this manuscript. This research was supported by funding from the National Institutes of Health Grants CA 49062 and ES 12888.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnson, G.E., Ivanov, V.N. & Hei, T.K. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 13, 790–802 (2008). https://doi.org/10.1007/s10495-008-0212-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0212-y