Abstract
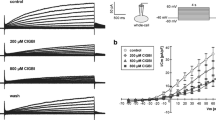
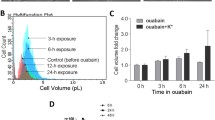
Plasma membrane potassium (K+) channels are required for tumor cell proliferation and apoptosis. However, the signal transduction mechanisms underlying K+ channel-dependent tumor cell proliferation or apoptosis remains elusive. Using HeLa and A2780 cells as study models, we tested the hypothesis that apoptotic proteins are linked with K+ channel-dependent tumor cell cycle and apoptosis. The patch-clamping study using the whole-cell mode revealed two components of voltage-gated outward K+ currents: one is sensitive to either tetraethylammonium (TEA) or tetrandrine (Tet), a maxi-conductance Ca2+-activated K+ (BK) channel blocker, and the other is sensitive to 4-aminopyridine (4-AP), a delayed rectifier K+ channel blocker. MTT and flow cytometry assays showed that TEA, Tet, or iberiotoxin (Ibtx), a selective BK channel blocker, inhibited HeLa and A2780 cell proliferation in a dose-dependent manner with G1 phase arrest. Pretreatment with TEA or Tet also induced apoptosis in HeLa and A2780 cells. However, glibenclamide (Gli), an ATP-sensitive K+ channel blocker, did not influence K+ currents, proliferation or apoptosis. Western blot analyses showed that while pretreatment of TEA and Tet produced an increase in expressions of p53, p21, and Bax, pretreatment of these two agents led to a decrease in expressions of heat shock protein (hsp)90α, hsp90β, and hsp70. Our results indicate that the blockade of BK channels results in tumor cell apoptosis and cycle arrest at G1 phase, and the transduction pathway underlying the anti-proliferative effects is linked to the increased expression of apoptotic protein p53 and the decreased expression of its chaperone proteins hsp.






Similar content being viewed by others
References
Wonderlin WF, Strobl JS (1996) Potassium channels, proliferation and G1 progression. J Membr Biol 154:91–107
Wang Z (2004) Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch 448:274–286
Kunzelmann K (2005) Ion channels and cancer. J Membr Biol 205:159–173
Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stuhmer W (2005) Role of voltage-gated potassium channels in cancer. J Membr Biol 205:115–124
Lang F, Foller M, Lang KS et al (2005) Ion channels in cell proliferation and apoptotic cell death. J Membr Biol 205:147–157
Wang L, Zhou P, Craig RW, Lu L (1999) Protection from cell death by mcl-1 is mediated by membrane hyperpolarization induced by K+ channel activation. J Membr Biol 172:113–120
Plummer H, Yu Q, Cakir Y, Schuller H (2004) Expression of inwardly rectifying potassium channels (GIRKs) and beta-adrenergic regulation of breast cancer cell lines. BMC Cancer 4:93–103
Liu SI, Chi CW, Lui WY et al (1998) Correlation of hepatocyte growth factor-induced proliferation and calcium-activated potassium current in human gastric cancer cells. Biochim Biophys Acta 1368:256–266
Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y (2002) Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci 22:1840–1849
Huang MH, Wu SN, Chen CP, Shen AY (2002) Inhibition of Ca2+-activated and voltage-dependent K+ currents by 2-mercaptophenyl-1,4-naphthoquinone in pituitary GH3 cells: contribution to its antiproliferative effect. Life Sci 70:1185–1203
Jager H, Dreker T, Buck A, et al (2004) Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol 65:630–638
Malhi H, Irani AN, Rajvanshi P et al (2000) KATP channels regulate mitogenically induced proliferation in primary rat hepatocytes and human liver cell lines. Implications for liver growth control and potential therapeutic targeting J Biol Chem 275:26050–26057
Ouadid-Ahidouch H, Chaussade F, Roudbaraki M et al (2000) KV1.1 K+ channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem Biophys Res Commun 278:272–277
Pardo LA, del Camino D, Sanchez A et al (1999) Oncogenic potential of EAG K(+) channels. Embo J 18:5540–7
Wang H, Zhang Y, Cao L et al (2002) HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res 62:4843–4848
Crociani O, Guasti L, Balzi M et al (2003) Cell cycle-dependent expression of HERG1 and HERG1B isoforms in tumor cells. J Biol Chem 278:2947–2955
Latorre R, Miller C (1983) Conduction and selectivity in potassium channels. J Membr Biol 71:11–30
Wu SN, Lo YK, Li HF, Shen AY (2001) Functional coupling of voltage-dependent L-type Ca2+ current to Ca2+-activated K+ current in pituitary GH3 cells. Chin J Physiol 44:161–167
Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC (2003) Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol 471:157–164
Ouadid-Ahidouch H, Roudbaraki M, Delcourt P et al (2004) Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol 287:C125–C134
Ransom CB, Sontheimer H (2001) BK channels in human glioma cells. J Neurophysiol 85:790–803
Ransom CB, Liu X, Sontheimer H (2002) BK channels in human glioma cells have enhanced calcium sensitivity. Glia 38:281–291
Weaver AK, Liu X, Sontheimer H (2004) Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res 78:224–234
DeFilippis RA, Goodwin EC, Wu L, DiMaio D (2003) Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol 77:1551–1563
Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P (2004) Prevarskaya N Cell-cycle-dependent expression of the large Ca2+-activated K+ channels in breast cancer cells. Biochem Biophys Res Commun 316:244–251
Akar F, Uydes-Dogan BS, Buharalioglu CK et al (1999) Protective effect of cromakalim and diazoxide, and proulcerogenic effect of glibenclamide on indomethacin-induced gastric injury. Eur J Pharmacol 374:461–470
Chrabi A, Horisberger JD (1999) Stimulation of epithelial sodium channel activity by the sulfonylurea glibenclamide. J Pharmacol Exp Ther 290:341–347
Wang G, Lemos JR (1992) Tetrandrine blocks a slow, large-conductance, Ca2+-activated potassium channel besides inhibiting a non-inactivating Ca2+ current in isolated nerve terminals of the rat neurohypophysis. Pflugers Arch 421:558–565
Wang G, Lemos JR (1995) Tetrandrine: a new ligand to block voltage-dependent Ca2+ and Ca2+-activated K+ channels. Life Sci 56:295–306
Wang G, Lemos JR, Iadecola C (2004) Herbal alkaloid tetrandrine: fron an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol Sci 25:120–123
Chen YJ (2002) Potential role of tetrandrine in cancer therapy. Acta Pharmacol Sin 23:1102–1106
Jin S, Levine AJ (2001) The p53 functional circuit. J Cell Sci 114:4139–4140
Gomez-Lazaro M, Fernandez-Gomez FJ, Jordan J (2004) p53: 25 years understanding the mechanism of genome protection. J Physiol Biochem 60:287–307
Zamzami N, Kroemer G (2005) p53 in apoptosis control: an introduction. Biochem Biophys Res Commun 331:685–687
O’Connor PM, Jackman J, Bae I et al (1997) Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res 57:4285–4300
Franco-Gou R, Rosello-Catafau J, Casillas-Ramirez A, Massip-Salcedo M, Rimola A, Calvo N, Bartrons R, Peralta C (2006) how ischaemic preconditioning protects small liver grafts. J Pathol 208:62–73
Shinohara T, Takahashi N, Ooie T, Ichinose M, Hara M, Yonemochi H, Saikawa T, Yoshimatsu H (2004) Estrogen inhibits hyperthermia-induced expression of heat-shock protein 72 and cardioprotection against ischemia/reperfusion injury in female rat heart. Mol Cell Cardiol 37:1053–1061
Kiang JG, Gist ID, Tsokos GC (2000) Regulation of heat shock protein 72 kDa and 90 kDa in human breast cancer MDA-MB-231 cells. Mol Cell Biochem 204:169–178
Neckers L, Ivy SP (2003) Heat shock protein 90. Curr Opin Oncol 15:419–424
Zylicz M, King FW, Wawrzynow A (2001) Hsp70 interactions with the p53 tumour suppressor protein. Embo J 20:4634–4638
Acknowledgments
This work was supported by grants from the National Science Foundation of China (No. 30571950, 30500596; 30626029; 30672227) and the “973” Program of China (No. 2002CB513100).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, X., Wang, F., Yao, W. et al. Heat shock proteins and p53 play a critical role in K+ channel-mediated tumor cell proliferation and apoptosis. Apoptosis 12, 1837–1846 (2007). https://doi.org/10.1007/s10495-007-0101-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0101-9