Abstract
Background
There is no standard chemotherapy available for unresectable or metastatic small bowel adenocarcinoma (SBA) because of its rarity. This systematic review aims to assess the efficacy and safety of chemotherapy for patients with unresectable or metastatic SBA.
Methods
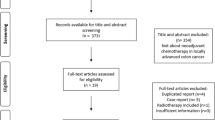
In accordance with the PRISMA statements, literature search was conducted using PubMed, Scopus, and the Cochrane Central Register of Controlled Trials. The included studies were prospective randomized, nonrandomized, or observational studies. Risk of bias was assessed the ROBINS-I tool.
Results
Seven prospective single-arm Phase II studies were included in this review. Six of them were assessed as having a moderate risk of bias and one as having a serious risk of bias. A meta-analysis was not performed, because the studies were single-arm. Systemic chemotherapy based on fluoropyrimidine regimens achieved favorable outcomes with acceptable adverse effects as a first therapy; however, the regimens differed in each study. The object response rate was 18–50%, and the disease control rate was 29–87%. With 5-fluorouracil, adriamycin, and mitomycin-C regimen, one treatment-related death occurred. A second line of therapy including chemotherapy with nab-paclitaxel also showed favorable efficacy. The object response rate was 20%, and the disease control rate was 50%.
Conclusions
Systemic chemotherapy based on fluoropyrimidine regimens was mainly used for unresectable or metastatic SBA. While it may achieve favorable outcomes with acceptable adverse effects, further evidence is needed.

Similar content being viewed by others
References
The Project Surveillance of Rare Cancers in Europe (RARECARE) RARECARE Cancer List n.d. https://www.rarecare.eu/rarecancers/rarecancers.asp. Accessed 20 Apr 2019
Raghav K, Overman MJ (2013) Small bowel adenocarcinomas-existing evidence and evolving paradigms. Nat Rev Clin Oncol 10:534–544. https://doi.org/10.1038/nrclinonc.2013.132
Verma D, Stroehlein JR (2006) Adenocarcinoma of the small bowel: a 60-yr perspective derived from MD Anderson cancer center tumor registry. Am J Gastroenterol 101:1647–1654. https://doi.org/10.1111/j.1572-0241.2006.00625.x
National Comprehensive Cancer Network (2019) NCCN clinical practice guidelines in oncology: colon cancer. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 20 Apr 2019
National Comprehensive Cancer Network (2019) NCCN clinical practice guidelines in oncology: small bowel adenocarcinoma. https://www.nccn.org/professionals/physician_gls/pdf/small_bowel.pdf. Accessed 12 Aug 2019
Ye X, Zhang G, Chen H et al (2018) Meta-analysis of postoperative adjuvant therapy for small bowel adenocarcinoma. PLoS ONE 13:e0200204. https://doi.org/10.1371/journal.pone.0200204
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700–b2700. https://doi.org/10.1136/bmj.b2700
Sterne JA, Hernán MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:4–10. https://doi.org/10.1136/bmj.i4919
Nordic Cochrane Centre The Cochrane Collaboration (2014) Review manager (RevMan) [computer program] Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen
Gibson MK, Holcroft CA, Kvols LK et al (2005) Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist 10:132–137. https://doi.org/10.1634/theoncologist.10-2-132
Overman MJ, Varadhachary GR, Kopetz S et al (2009) Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of vater. J Clin Oncol 27:2598–2603. https://doi.org/10.1200/JCO.2008.19.7145
Xiang XJ, Liu YW, Zhang L et al (2012) A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs 23:561–566. https://doi.org/10.1097/CAD.0b013e328350dd0d
Horimatsu T, Nakayama N, Moriwaki T et al (2017) A phase II study of 5-fluorouracil/L-leucovorin/oxaliplatin (mFOLFOX6) in Japanese patients with metastatic or unresectable small bowel adenocarcinoma. Int J Clin Oncol 22:905–912. https://doi.org/10.1007/s10147-017-1138-6
Gulhati P, Raghav K, Shroff RT et al (2017) Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: a single-center, open-label, phase 2 study. Cancer 123:1011–1017. https://doi.org/10.1002/cncr.30445
McWilliams RR, Foster NR, Mahoney MR et al (2017) North central cancer treatment group N0543 (Alliance): a phase 2 trial of pharmacogenetic-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer 123:3494–3501. https://doi.org/10.1002/cncr.30766
Overman MJ, Adam L, Raghav K et al (2018) Phase II study of nab-paclitaxel in refractory small bowel adenocarcinoma and CpG island methylator phenotype (CIMP)-high colorectal cancer. Ann Oncol 29:139–144. https://doi.org/10.1093/annonc/mdx688
Gulhati P, Raghav K, Shroff R et al (2017) Phase II study of panitumumab in RAS wild-type metastatic adenocarcinoma of small bowel or ampulla of vater. Oncologist. https://doi.org/10.1634/theoncologist.2017-0568
National Cancer Institute. Common terminology criteria for adverse events n.d. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 8 Apr 2019
Singhal N, Singhal D (2007) Adjuvant chemotherapy for small intestine adenocarcinoma. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005202.pub2
Dabaja BS, Suki D, Pro B et al (2004) Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer 101:518–526. https://doi.org/10.1002/cncr.20404
Howe JR, Karnell LH, Menck HR et al (1999) Adenocarcinoma of the small bowel. Cancer 86:2693–2706. https://doi.org/10.1002/(SICI)1097-0142(19991215)86:12%3c2693:AID-CNCR14%3e3.0.CO;2-U
Overman MJ, Hu C-Y, Wolff RA et al (2010) Prognostic value of lymph node evaluation in small bowel adenocarcinoma. Cancer 116:5374–5382. https://doi.org/10.1002/cncr.25324
Brierley JD, Gospodarowicz MK, Wittekind C (eds) (2017) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell
Honma Y, Ueno M, Kanemitsu Y et al (2018) Randomized phase III study of observation versus adjuvant capecitabine and oxaliplatin in curatively resected small bowel adenocarcinoma: a Japan Clinical Oncology Group study (JCOG1502C). J Clin Oncol 36:TPS539. https://doi.org/10.1200/JCO.2018.36.4_suppl.TPS539
Bogaerts J, Sydes MR, Keat N et al (2015) Clinical trial designs for rare diseases: studies developed and discussed by the international rare cancers initiative. Eur J Cancer 51:271–281. https://doi.org/10.1016/j.ejca.2014.10.027
Acknowledgements
YN was supported by Japan Society for the Promotion of Science.
Funding
This work was not supported by a specific funding.
Author information
Authors and Affiliations
Contributions
YN, NH, TH: Conception and design; YN, NH: Literature search and data analysis; all authors: Interpretation of the results; YN: Drafting of the article; all authors: Critical revision of the article f; all authors: Final approval of the article.
Corresponding author
Ethics declarations
Conflict of interest
MM received research funding outside the submitted work from TAIHO PHARMACEUTICAL CO., LTD., Nissha Co., Ltd., KUBIX Inc., SYSMEX CORPORATION, Thyas Co., Ltd., ONO PHARMACEUTICAL CO., LTD., ACTmed Co., LTD., Eisai Co., Ltd., RIKEN GENESIS CO., LTD., CHUGAI PHARMACEUTICAL CO., LTD., Bio-xcelerator K.K., Gtheranostics Co., Ltd., Yakult Honsha Co., Ltd., IQVIA Services Japan K.K., Parexel International Inc., MSD K.K., and Meiji Seika Pharma Co., Ltd; and scholarship donation outside the submitted work from ARK INNOVATION Inc., ONO PHARMACEUTICAL CO., LTD, AYUMI Pharmaceutical Corporation, Showa Yakuhin Kako Co.,Ltd., CHUGAI PHARMACEUTICAL CO., LTD., INTAGE Healthcare Inc., Cyber Laboratory Inc., Gtheranostics Co., Ltd., and Yakult Honsha Co.,Ltd. TN received honoraria outside the submitted work from ONO PHARMACEUTICAL CO., LTD., Nippon Boehringer Ingelheim Co., Ltd., and Pfizer Japan Inc. YN, NH, TH, TF, KH, and YS declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Nishikawa, Y., Hoshino, N., Horimatsu, T. et al. Chemotherapy for patients with unresectable or metastatic small bowel adenocarcinoma: a systematic review. Int J Clin Oncol 25, 1441–1449 (2020). https://doi.org/10.1007/s10147-020-01703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01703-z