Abstract
Background
To assess the usefulness of positron emission tomography combined with computed tomography using 18F-fluorodeoxyglucose (FDG PET/CT) for optimizing chemotherapy during neoadjuvant chemotherapy for primary breast cancer.
Methods
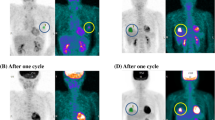
One hundred and eight patients (110 tumors) with breast cancer (≥2 cm, stages II and III) received neoadjuvant chemotherapy consisting of an anthracycline-based regimen and taxane. The maximal value of the baseline standardized uptake value (SUV) and the change in SUV after four cycles of an anthracycline-based regimen relative to baseline SUV were assessed for predicting pathological complete response (pCR) after sequential taxane.
Results
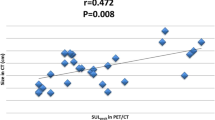
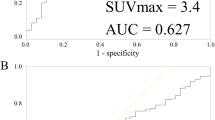
Tumors with pCR had significantly higher baseline SUV (9.3 ± 3.7 SD) compared to those with non-pCR (7.2 ± 3.8 SD) (p = 0.02), but there was a considerable overlap between two groups. On PET scan after four cycles of chemotherapy, thirty-three patients (33.7%) with a 72.1% or greater reduction in SUV were considered as responders and the performance in predicting pCR had a sensitivity of 88.9% and specificity of 78.7%.
Conclusion
The baseline SUV could not be a useful indicator for predicting pCR due to the wide range in sensitivity. On the other hand, a relative change in SUV after completion of an anthracycline-based regimen could be useful for predicting pCR.



Similar content being viewed by others
References
De Laurentiis M, Cancello G, Zinno L et al (2005) Targeting HER2 as a therapeutic strategy for breast cancer: a paradigmatic shift of drug development in oncology. Ann Oncol 16(Suppl 4):iv7–iv13
Ferguson T, Wilcken N, Vagg R et al (2007) Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev 2007(4):CD004421
Henderson IC, Berry DA, Demetri GD et al (2003) Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21(6):976–983
Mamounas EP, Bryant J, Lembersky B et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23(16):3686–3696
Sachelarie I, Grossbard ML, Chadha M et al (2006) Primary systemic therapy of breast cancer. Oncol 11(6):574–589
Kaufmann M, von Minckwitz G, Bear HD et al (2007) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 18(12):1927–1934
Caudle AS, Gonzalez-Angulo AM, Hunt KK et al (2010) Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(11):1821–1828
van de Vijver MJ, He YD, van’t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347(25):1999–2009
Juweid ME, Cheson BD (2006) Positron-emission tomography and assessment of cancer therapy. N Engl J Med 354(5):496–507
Young H, Baum R, Cremerius U et al (1999) Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 35(13):1773–1782
Shankar LK, Hoffman JM, Bacharach S et al (2006) Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med 47(6):1059–1066
Avril N, Rose CA, Schelling M et al (2000) Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 18(20):3495–3502
Schelling M, Avril N, Nahrig J et al (2000) Positron emission tomography using [(18)F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol 18(8):1689–1695
Rousseau C, Devillers A, Sagan C et al (2006) Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol 24(34):5366–5372
Smith IC, Welch AE, Hutcheon AW et al (2000) Positron emission tomography using [(18)F]-fluorodeoxy-d-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 18(8):1676–1688
Schwarz-Dose J, Untch M, Tiling R et al (2009) Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol 27(4):535–541
Beresford MJ, Wilson GD, Makris A (2006) Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res 8(6):216
Podoloff DA, Ball DW, Ben-Josef E et al (2009) NCCN task force: clinical utility of PET in a variety of tumor types. J Natl Compr Canc Netw 7(Suppl 2):1–26
Ueda S, Tsuda H, Asakawa H et al (2008) Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol 38(4):250–258
Ueda S, Kondoh N, Tsuda H et al (2008) Expression of centromere protein F (CENP-F) associated with higher FDG uptake on PET/CT, detected by cDNA microarray, predicts high-risk patients with primary breast cancer. BMC Cancer 8:384
Ueda S, Tsuda H, Saeki T et al (2010) Early metabolic response to neoadjuvant letrozole, measured by FDG PET/CT, is correlated with a decrease in the Ki67 labeling index in patients with hormone receptor-positive primary breast cancer: a pilot study. Breast Cancer. doi:10.1007/s12282-010-0212-y
Ito K, Kubota K, Morooka M et al (2009) Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med 23(7):671–676
Kurosumi M, Akashi-Tanaka S, Akiyama F et al (2008) Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer (Tokyo, Japan) 15(1):5–7
Hayes DF, Thor AD, Dressler LG et al (2007) HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med 357(15):1496–1506
Bedard PL, Di Leo A, Piccart-Gebhart MJ (2010) Taxanes: optimizing adjuvant chemotherapy for early-stage breast cancer. Nat Rev 7(1):22–36
Buzdar AU, Ibrahim NK, Francis D et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Buzdar AU, Valero V, Ibrahim NK et al (2007) Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res 13(1):228–233
Straver ME, Aukema TS, Olmos RA et al (2010) Feasibility of FDG PET/CT to monitor the response of axillary lymph node metastases to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med Mol Imag 37(6):1069–1076
Ueda S, Tsuda H, Asakawa H et al (2008) Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer 8:165
Acknowledgments
This study was supported by the grants of Department of Breast Oncology, Saitama Medical University and Medical Research of National Defense Force.
Conflict of interest
No authors has any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ueda, S., Saeki, T., Shigekawa, T. et al. 18F-Fluorodeoxyglucose positron emission tomography optimizes neoadjuvant chemotherapy for primary breast cancer to achieve pathological complete response. Int J Clin Oncol 17, 276–282 (2012). https://doi.org/10.1007/s10147-011-0287-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0287-2