Abstract
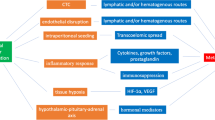
General anesthesia accompanied by surgical stress is considered to suppress immunity, presumably by directly affecting the immune system or activating the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Along with stress such as surgery, blood transfusion, hypothermia, hyperglycemia, and postoperative pain, anesthetics per se are associated with suppressed immunity during perioperative periods because every anesthetic has direct suppressive effects on cellular and neurohumoral immunity through influencing the functions of immunocompetent cells and inflammatory mediator gene expression and secretion. Particularly in cancer patients, immunosuppression attributable to anesthetics, such as the dysfunction of natural killer cells and lymphocytes, may accelerate the growth and metastases of residual malignant cells, thereby worsening prognoses. Alternatively, the anti-inflammatory effects of anesthetics may be beneficial in distinct situations involving ischemia and reperfusion injury or the systemic inflammatory response syndrome (SIRS). Clinical anesthesiologists should select anesthetics and choose anesthetic methods with careful consideration of the clinical situation and the immune status of critically ill patients, in regard to long-term mortality, morbidity, and the optimal prognosis.
Similar content being viewed by others
References
Graham EA. The influence of ether and ether anesthesia on bacteriolysis, agglutination and phagocytosis. J Infect Dis. 1911;8:147.
Gaylord HR, Simpson BT. Effect of certain anaesthetics and loss of blood upon growth of transplanted mouse cancer. J Cancer Res. 1916;1:379–382.
Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol. 2006;19:423–428.
Vallejo R, Hord ED, Barna SA, Santiago-Palma J, Ahmed S. Perioperative immunosuppression in cancer patients. J Environ Pathol Toxicol Oncol. 2003;22:139–146.
Chrousos GP. Seminars in medicine of the Beth Israel Hospital, Boston: the hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362.
Kennedy BC, Hall GM. Neuroendocrine and inflammatory aspects of surgery: do they affect outcome? Acta Anaesthesiol Belg. 1999;50:205–209.
Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann NY Acad Sci. 2002;966:290–303.
Younes RN, Rogatko A, Brennan MF. The influence of intraoperative hypotension and perioperative blood transfusion on disease-free survival in patients with complete resection of colorectal liver metastases. Ann Surg. 1991;214:107–113.
Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden J, Adson MA. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504.
Tatter PI. Perioperative blood transfusion and colorectal cancer: a review. J Surg Oncol. 1988;39:197–200.
Rassias AJ, Marrin CAS, Arruda J, Whalen PK, Beach M, Yeager MP. Insulin infusion improves neutrophil function in diabetic cardiac surgery patients. Anesth Analg. 1999;88:1011–1016.
Rassias AJ, Givan AL, Marrin CAS, Whalen K, Pahl J, Yearger MP. Insulin increases neutrophil count and phagocytosis capacity after cardiac surgery. Anesth Analg. 2002;94:1113–1119.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–1215.
Beilin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H. Effect of mild perioperative hypothermia on cellular immune responses. Anesthesiology. 1998;89:1133–1140.
Sheffield CW, Sessler DI, Hunt TK. Mild hypothermia during isoflurance anesthesia decreases resistance to E. coli dermal infection in guinea pigs. Acta Anaesthesiol Scand. 1994;38:201–205.
Beilin B, Shavit Y, Trabekin E, Mordashev B, Mayburd E, Zeidel A, Bessler H. The effects of postoperative pain management on immune response to surgery. Anesth Analg. 2003;97:822–827.
Volk T, Schenk M, Voigt K, Tohtz S, Putzier M, Kox WJ. Postoperative epidural anesthesia preserves lymphocyte, but not monocyte, immune function after major spine surgery. Anesth Analg. 2004;98:1086–1092.
Yokoyama M, Itano Y, Mizobuchi S. The effects of epidural block on the distribution of lymphocyte subsets and natural-killer cell activity in patients with and without pain. Anesth Analg. 2001;92:463–469.
Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24:690–695.
Black CT, Hennessey PJ, Andrassy RJ. Short-term hyperglycemia depresses immunity through nonenzymatic glycosylation of circulating immunoglobulin. J Trauma. 1990;30:830–833.
Wilson RM. Neutrophil function in diabetes. Diabetic Med. 1986;3:509–512.
Wilson RM, Tomlinson DR, Reeves WG. Neutrophil sorbitol production impairs oxidative killing in diabetes. Diabetic Med. 1987;4:37–40.
Kirkley SA, Cowles J, Pellegrini VD Jr, Harris CM, Boyd AD, Blumberg N. Cytokine secretion after allogeneic or autologous blood transfusion (letter). Lancet. 1995;345:527.
Kirkley S, Cowles J, Pellegrini V, Harris C, Boyd A, Blumberg N. Increased T helper 2 (TH2) type cytokine secretion found in surgical patients receiving allogeneic blood. Transfusion. 1995;35(Suppl):44.
Kelbel I, Weiss M. Anesthetics and immune function. Curr Opin Anaesthesiol. 2001;14:685–691.
Benjamini E, Coico R, Sunshine G. Elements of innate and acquired immunity. In: Benjamini E, Coico R, Sunshine G, editors. Immunology—a short course. New York: Wiley-Liss; 2000, p. 17–39.
Benjamini E, Coico R, Sunshine G. Biology of the T lymphocyte. In: Benjamini E, Coico R, Sunshine G, editors. Immunology—a short course. New York: Wiley-Liss; 2000. p. 169–185.
Weiss A. T-lymphocyte activation. In: Paul WE, editor. Fundamental immunology. Philadelphia: Lippincott-Raven; 1999. p. 411–448.
Mack VE, McCarter MD, Naama HA, Calvano SE, Daly JM. Dominance of T helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303–1309.
Powrie F, Coffman RL. Cytokine regulation of T cell function: potential for therapeutic intervention. Immunol Today. 1993;14:270–274.
Yokoyama WM. Natural killer cells. In: Paul WE, editor. Fundamental immunology. Philadelphia: Lippincott-Raven; 1999. p. 575–604.
Schreiber H. Tumor immunology. In: Paul WE, editor. Fundamental immunology. Philadelphia: Lippincott-Raven; 1999. p. 1237–1270.
Kurosawa S, Matsuzaki G, Harada M, Ando Takashi, Nomoto K. Early appearance and activation of natural killer cells in tumor-infiltrating lymphoid cells during tumor development. Eur J Immunol. 1993;23:1029–1033.
Kurosawa S, Harada M, Matsuzaki G, Shinomiya H, Terao H, Kobayashi N, Nomoto K. Early appearing tumour-infiltrating natural killer cells play a crucial role in the generation of antitumour T lymphocytes. Immunology. 1995;85:338–346.
Kurosawa S, Harada M, Shinomiya Y, Terao H, Nomoto K. The concurrent administration of OK432 augments the antitumor vaccination effect with tumor cells by sustaining locally infiltrating natural killer cells. Cancer Immunol Immunother. 1996;43:31–38.
Kos FJ, Engelman EG. Immune regulation: a critical link between NK cells and CTLs. Immunol Today. 1996;17:174–176.
Trinchieri G. Biology of natural killer cells. In: Dixon FJ, editor. Advances in immunology. San Diego: Academic; 1989. p. 187–376.
Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Cutting edge: differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–5824.
Seo N, Tokura Y. Downregulation of innate and acquired antitumor immunity by bystander gammadelta and alphabeta T lymphocytes with Th2 or Tr1 cytokine profiles. J Interferon Cytokine Res. 1999;19:555–561.
Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888.
Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Rev Immunol. 2006;6:173–182.
Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2006;16:87–92.
Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376.
Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497.
Welch WD. Halothane reversibly inhibits human neutrophil bacterial killing. Anesthesiology. 1981;55:650–654.
Nakagawara M, Takeshige K, Takamatsu J, Takahashi S, Yoshitake J, Minakami S. Inhibition of superoxide production and Ca2+ mobilization in human neutrophils by halothane, enflurane, and isoflurane. Anesthesiology. 1986;64:4–12.
Fröhlich D, Rothe G, Schwall B, Schmid P, Schmitz G, Taeger K, Hobbhahn J. Effects of volatile anaesthetics on human neutrophil oxidative response to the bacterial peptide FMLP. Br J Anaesth. 1997;78:718–723.
Guochang H, Salem MR, Crystal GJ. Isoflurane prevents platelets from enhancing neutrophil-induced coronary endothelial dysfunction. Anesth Analg. 2005;101:1261–1268.
Fan H, Sun B, Gu Q, Lafond-Walker A, Cao S, Becker LC. Oxygen radicals trigger activation of NF-κ B and AP-1 and upregulation of ICAM-1 in reperfused canine heart. Am J Physiol. 2002;282:H1778–H1786.
Hu G, Vinten-Johansen J, Salem MR, Zhao ZQ, Crystal GJ. Isoflurane inhibits neutrophil-endothelium interactions in the coronary circulation: lack of role for adenosine triphosphate-sensitive potassium channels. Anesth Analg. 2002;94:849–856.
Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res. 1999;43:860–878.
De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: Mechanisms and clinical implications. Anesth Analg. 2005;100:1584–1593.
Kevin LG, Novalija E, Stowe DF. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg. 2005;101:1275–1287.
Tait AR, Davidson BA, Johnson KJ, Remick DG, Knight PR. Halothane inhibits the intraalveolar recruitment of neutrophils, lymphocytes, and macrophages in response to influenza virus infection in mice. Anesth Analg. 1993;76:1106–1113.
Kotani N, Hashimoto H, Sessler DI, Kikuchi A, Suzuki A, Takahashi S, et al. Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1125–1132.
Boost KA, Flondor M, Hofstetter C, Platacis I, Stegewerth K, Hoegl S, et al. The beta-adrenoceptor antagonist propranolol counteracts anti-inflammatory effects of isoflurane in rat endotoxemia. Acta Anaesthesiol Scand. 2007;51:900–908.
Tschaikowsky K, Ritter J, Schröppel K, Kühn M. Volatile anesthetics differentially affect immunostimulated expression of inducible nitric oxide synthase: role of intracellular calcium. Anesthesiology. 2000;92:1093–1102.
Wallace JL. Nitric oxide as a regulator of inflammatory process. Mem Inst Oswaldo Cruz. 2005;100:5–9.
Chello M, Mastroroberto P, Marchese A, Maltese G, Santangelo E, Amantea B. Nitric oxide inhibits neutrophil adhesion during experimental extracorporeal circulation. Anesthesiology. 1998;89:443–448.
Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–517.
Fuentes JM, Talamini MA, Fulton WB, Hanly EJ, Aurora AR, Demaio A. Genaral anesthesia delays the inflammatory response and increases survival for mice with endotoxic shock. Clin Vaccine Immunol. 2006;13:281–288.
Hofstetter C, Flondor M, Boost KA, Koehler P, Bosmann M, Pfeilschifter J, Zwissler B, Mühl H. A brief exposure to isoflurane (50 s) significantly impacts on plasma cytokine levels in endotoxemic rats. Int Immunopharmacol. 2005;5:1519–1522.
Hofstetter C, Boost KA, Flondor M, Basagan-Mogol E, Betz C, Homann M, Mühl H, Pfeilschifter J, Zwissler B. Anti-inflammatory effects of sevoflurane and mild hypothermia in endotoxemic rats. Acta Anaesthesiol Scand. 2007;51:893–899.
Tarter PI, Steinberg B, Barron DM, Martinelli G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg. 1987;122:1264–1268.
Schantz SP, Brown BW, Lisa E, Taylor DL, Beddingfield N. Evidence for the role of natural immunity in the control of metastatic spread of head and neck cancer. Cancer Immunol Immunother. 1987;25:141–145.
Fujiwara T, Yamaguchi Y. Autologous tumor killing activity as a prognostic factor in primary resected nonsmall cell carcinoma of the lung. Cancer. 1997;79:474–481.
Woods GM, Griffiths DM. Reversible inhibition of natural killer cell activity by volatile anaesthetic agents in vitro. Br J Anaesth. 1986;58:535–539.
Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78:700–706.
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–1339.
Markovic SN, Murasko DM. Anesthesia inhibits interferon-induced natural killer cell cytotoxicity via induction of CD8+ suppressor cells. Cell Immunol. 1993;151:474–480.
Tønnesen E, Brinkløv MM, Christensen NJ, Olesen AS, Madsen T. Natural killer cell activity and lymphocyte function during and after coronary artery bypass grafting in relation to the endocrine stress response. Anesthesiology. 1987;67:526–533.
Salo M. Effects of anaesthesia and surgery on the immune response. In: Watkins J, Salo M, editors. Trauma, stress and immunity in anaesthesia and surgery. London: Butterworth Scientific; 1982. p. 211–253.
Salo M, Eskola J, Nikoskelainen J. T-and B-lymphocyte function in anesthetics. Acta Anaesthesiol Scand. 1984;28:292–295.
Bruce DL. Halothane inhibition of phytohemagglutinin-induced transformation of lymphocytes. Anesthesiology. 1972;36:201–205.
Bruce DL. Halothane inhibition of RNA and protein synthesis of PHA-treated human lymphocytes. Anesthesiology. 1975;42:11–14.
Ferrero E, Ferrero ME, Marni A, Zocchi MR, Stella L, Rugarli C, Tiengo M. In vitro effects of halothane on lymphocytes. Eur J Anaesthesiol. 1986;3:321–330.
Hamra JG, Yaksh TL. Halothane inhibits T cell proliferation and interleukin-2 receptor expression in rats. Immunopharmacol Immunotoxicol. 1996;18:323–336.
Stevenson GM, Hall SC, Miller PJ, Alvord G, Leventhal JB, Seleny F, Stevenson HC. The effects of anesthetic agents on human immune system function. I. Design of a system to deliver inhalational anesthetic agents to leukocytes cultures in vitro. J Immunol Methods. 1986;88:277–283.
Mitsuhata H, Shimizu R, Yokoyama MM. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int J Immunopharmacol. 1995;17:529–534.
Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95:1467–1472.
Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl H, Geiger KK, Pannen BHJ. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–1157.
Green DR. Overview: apoptotic signaling pathway in the immune system. Immunol Rev. 2003;193:5–9.
M’Bemba-Meka P, Lemieux N, Chakrabarti SK. Role of oxidative stress, mitochondrial membrane potential, and calcium homeostasis in nickel subsulfide-induced human lymphocyte death in vitro. Sci Total Environ. 2006;369:21–34.
Le SB, Hailer MK, Buhrow S, Wang Q, Flatten K, Pediaditakis P, Bible KC, Lewis LD, Sausville EA, Pang YP, Ames MM, Lemasters JJ, Holmuhamedov EL, Kaufmann SH. Inhibition of mitochondrial respiration as a source of adaphostin-induced reactive oxygen species and cytotoxicity. J Biol Chem. 2007;282:8860–8872.
Kasahara Y, Iwai K, Yachie A, Ohta K, Konno A, Seki H, Miyazaki T, Taniguchi N. Involvement of reactive oxygen intermediates in spontaneous and CD95 (Fas/APO-1)-mediated apoptosis of neutrophils. Blood. 1997;89:1748–1753.
Gwinn M, Vallyathan V. Respiration burst: role in signal transduction in alveolar macrophages. J Toxicol Environ Health Part B. 2006;9:27–39.
Loop T, Scheiermann P, Doviakue D, Musshoff F, Humar M, Roesslein M, Hoetzel A, Schmidt R, Madea B, Geiger K, Pahl H, Pannen BH. Sevoflurane inhibits phorbol-myristate-acetate-induced activator protein-1 activation in human T lymphocytes in vitro: potential role of the p38-stress kinase pathway. Anesthesiology. 2004;101:710–721.
De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg. 2005;100:1584–1593.
Zaugg M, Schaub M. Signaling and cellular mechanisms in cardiac protection by ischemic and pharmacological preconditioning. J Muscle Res Cell Motil. 2003;24:219–249.
Aarts L, van der Hee R, Dekker I, de Jong J, Langermeiger H, Bast A. The widely used anesthetic agent propofol can replace α-tocopherol as an antioxidant. FEBS Lett. 1995;357:83–85.
Heine J, Leuwer M, Scheinichen D, Arseniev L, Jaeger K, Piepenbrock S. Flow cytometry evaluation of the in vitro influence of four i.v. anaesthetics on respiratory burst of neutrophils. Br J Anaesth. 1996;77:387–392.
Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, Niwa Y. Propofol inhibits human neutrophil functions. Anesth Analg. 1998;87:695–700.
Heller A, Heller S, Blecken S, Urbaschek R, Koch T. Effects of intravenous anesthetics on bacterial elimination in human blood in vitro. Acta Anaesthesiol Scand. 1998;42:518–526.
Krumholz W, Endrass J, Hempelmann G. Propofol inhibits phagocytosis and killing of Staphylococcus aureus and Escherichia coli by polymorphonuclear leukocytes in vitro. Can J Anaesth. 1994;41:446–449.
Heine J, Jaeger K, Osthaus A, Weingaertner N, Munte S, Piepenbrock S, Leuwer M. Anaesthesia with propofol decreases FMLP-induced neutrophil respiratory burst but not phagocytosis compared with isoflurane. Br J Anaesth. 2000;85:424–430.
Davidson JA, Boom SJ, Pearsall FJ, Zhang P, Ramasay G. Comparison of the effects of four i.v. anaesthetic agents on polymorphonuclear leukocyte function. Br J Anaesth. 1995;74:315–318.
O’Donnell NG, McSharry CP, Wilkinson PC, Asbury AJ. Comparison of the inhibitory effects of propofol, thiopentone and midazolam on neutrophil polarization in vitro in the presence or absence of human serum albumin. Br J Anaesth. 1992;69:70–74.
Huettemann E, Jung A, Vogelsang H, Hou N, Sakka SG. Effects of propofol vs methohexital on neutrophil function and immune status in critically ill patients. J Anesth. 2006;20:86–91.
Galley HF, Dubbels AM, Webster NR. The effects of midazolam and propofol on interleukin-8 from human polymorphonuclear leukocytes. Anesth Analg. 1998;86:1289–1293.
Nagata T, Kansha M, Irita K, Takahashi S. Propofol inhibits FMLP-stimulated phosphorylation of p42 mitogen-activated protein kinase and chemotaxis in human neutrophils. Br J Anaesth. 2001;86:853–858.
Wu GJ, Tai YT, Chen TL, Lin LL, Ueng YF, Chen RM. Propofol specifically inhibits mitochondrial membrane potential but not complex I NADH dehydrogenase activity, thereby reducing cellular ATP biosynthesis and migration of macrophages. Ann NY Acad Sci. 2005;1042:168–176.
Chen RM, Wu CH, Chang HC, Wu GJ, Lin YL, Sheu JR, Chen TL. Propofol suppresses macrophage functions and modulates mitochondrial membrane potential and cellular adenosine triphosphate synthesis. Anesthesiology. 2003;98:1178–1185.
Chang H, Tsai SY, Chang Y, Chen TL, Chen RM. Therapeutic concentration of propofol protects mouse macrophages from nitric oxide-indiced cell death and apoptosis. Can J Anaesth. 2002;49:477–480.
Chen RM, Wu GJ, Tai YT, Sun WZ, Lin YL, Jean WC, Chen TL. Propofol reduces nitric oxide biosynthesis in lipopolysaccharide-activated macrophages by downregulating the expression of inducible nitric oxide synthase. Arch Toxicol. 2003;77:418–423.
Chen RM, Chen TG, Chen TL, Lin LL, Chang CC, Chang HC, Wu CH. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann NY Acad Sci. 2005;1042:262–271.
Rossano F, Tufano R, Cipollaro de L’Ero G, Servillo G, Baroni A, Tufano MA. Anesthetic agents induce human mononuclear leucocytes to release cytokines. Immunopharmacol Immunotoxicol. 1992;14:439–450.
Brand JM, Frohn C, Luhm J, Kirchner H, Schmucker P. Early alterations in the number of circulating lymphocyte subpopulations and enhanced proinflammatory immune response during opioid-based general anesthesia. Shock. 2003;20:213–217.
Pirttinkangas CO, Perttila J, Salo M. Propofol emulsion reduces proliferative responses of lymphocytes from intensive care patients. Intensive Care Med. 1993;19:299–302.
Devlin EG, Clarke RS, Mirakhur RK, McNeill TA. Effect of four i.v. induction agents on T-lymphocyte proliferations to PHA in vitro. Br J Anaesth. 1994;73:315–317.
Salo M, Pirttikangas CO, Pulkki K. Effects of propofol emulsion and thiopentone on T helper cell type-1/type-2 balance in vitro. Anesthesia. 1997;52:341–344.
Song HK, Jeong DC. The effect of propofol on cytotoxicity and apoptosis of lipopolysaccharide-treated mononuclear cells and lymphocytes. Anesth Analg. 2004;98:1724–1728.
Mozrzmas JW, Teisseyre A, Vittur F. Propofol blocks voltagegated potassium channels in human T lymphocytes. Biochem Pharmacol. 1996;52:843–849.
Loop T, Liu Z, Humar M, Hoetzel A, Benzing A, Pahl HL, Geiger KK, Pannen BHJ. Thiopental inhibits the activation of nuclear factor κB. Anesthesiology. 2002;96:1202–1213.
Larsen B, Hoff G, Wilhelm W, Buchinger H, Wanner G, Bauer M. Effect of intravenous anesthetics on spontaneous and endotoxin-stimulated cytokine response in cultured human whole blood. Anesthesiology. 1998;89:1218–1227.
Carr DJ, Rogers TJ, Weber RJ. The relevance of opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med. 1996;213:248–257.
Flores LR, Dretchen KL, Bayer BM. Potential role of the autonomic nervous system in the immunosuppressive effects of the acute morphine administration. Eur J Pharmacol. 1996;318:437–446.
Freier DO, Fucks BA. A mechanism of action for morphine induced immunosuppression: corticosterone mediates morphine induced suppression of NK cell activity. J Pharmacol Exp Ther. 1993;270:1127–1133.
Bryanyt HU, Bernton EW, Kenner JR, Holaday JW. Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology. 1991;128:3253–3258.
Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanisms of action. J Neuroimmunol. 1998;83:19–28.
Smith EM. Opioid peptides in immune cells. Adv Exp Med Biol. 2003;521:51–68.
Sacerdote P, Limiroli E, Gaspani L. Experimental evidence for imunomodulatory effects of opioids. Adv Exp Med Biol. 2003;521:106–116.
Welters ID, Fimiani C, Bilfinger TV, Stefano GB. NF-κB, nitric oxide and opiate signaling. Med Hypotheses. 2000;54:263–268.
Welters ID, Menzebach A, Goumon Y, Langefeld TW, Teschemacher H, Hempelmann G, Stefano BG. Morphine suppresses complement receptor expression, phagocytosis, and respiratory burst in neutrophils by a nitric oxide and mu(3) opiate receptor-dependent mechanism. J Neuroimmunol. 2000;111:139–145.
Roy S, Ramakrishnan S, Loh HH, Lee NM. Chronic morphine treatment selectively suppresses macrophage colony formation in bone marrow. Eur J Pharmacol. 1991;195:359–363.
Eisenstein TK, Hillburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell population. J Neuroimmunol. 1998;83:36–44.
Yeager MP, Colacchio TA, Yu CT, Hildebrandt L, Howell AL, Weiss J, Guyre PM. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83:500–508.
Bryant HU, Roudebush RE. Suppressive effects of morphine pellet implants on in vivo parameters of immune function. J Pharmacol Exp Ther. 1990;255:410–414.
Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-induced alterations of immune status: dose dependency, compartment specificity and antagonism by naltrexone. J Pharmacol Exp Ther. 1993;265:1071–1078.
Roy S, Charboneau RG, Barke RA. Morphine synergizes with lipopolysaccharide in a chronic endotoxemia model. J Neuroimmunol. 1999;95:107–114.
Casalinuovo IA, Graziano R, Di Francesco P. Cytokine secretion by murine spleen cells after inactivated Candida albicans immunization. Efect of cocaine and morphine treatment. Immunopharmacol Immunotoxicol. 2000;22:35–48.
Yin D, Mufson RA, Wang R, Shi Y. Fas-mediated cell death promoted by opioids. Nature. 1999;397:218.
Jaeger K, Scheinichen D, Heine J, Andre M, Bund M, Piepenbrock S, Leuwer M. Remifentanil, fentanyl and alfentanil have no effect on the respiratory burst of neutrophils in vitro. Acta Anaesthesiol Scand. 1998;42:1110–1113.
Krumholz W, Endrass J, Hemplemann G. Inhibition of phagocytosis and killing of bacteria by anaesthetic agents in vitro. Br J Anaesth. 1995;75:66–70.
Shavit Y, Ben-Eliyahu S, Zeidel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metstasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11:255–260.
Yeager MP, Procopio MA, DeLeo JA, Arruda JL, Hildebrandt L, Howell AL. Intravenous fentanyl increases natural killer cell cytotoxicity and circulating CD16+ lymphocytes in humans. Anesth Analg. 2002;94:94–99.
Jacobs R, Karst M, Scheinichen D, Bevilacqua C, Schneider Udo, Heine J, Schedlowski M, Schmidt RE. Effects of fentanyl on cellular immune functions in man. Int J Immunopharmacol. 1999;21:445–454.
Bilfinger TV, Fimiani C, Stefano GB. Morphine’s immunoregulatory actions are not shared by fentanyl. Int J Cardiol. 1998;64 (Suppl 1):S61–S66.
Tønnesen E, Wahlgreen C. Influence of extradural and general anaesthesia on natural killer cell activity and lymphocyte subpopulations in patients undergoing hysterectomy. Br J Anaesth. 1988;60:500–507.
Høgevold HE, Lyberg T, Kähler H, Haug E, Reikerås O. Changes in plasma IL-1-βTNF-α and IL-6 after total hip replacement surgery in general or regional anesthesia. Cytokine. 2000;12:1156–1159.
Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24:690–695.
Hole A, Unsgaard G. The effect of epidural and general anaesthesia on lymphocyte functions during and after major orthopaedic surgery. Acta Anaesthesiol Scand. 1983;27:135–141.
Whelan P, Morris PJ. Immunological responsiveness after transurethral resection of the prostate: general versus spinal anaesthetic. Clin Exp Immunol. 1982;48:611–618.
Wada H, Seki S, Takahahi T, Kawarabayashi N, Higuchi H, Habu Y, Sugahara S, Kazama T. Combined spinal and general anesthesia attenuates liver metastasis by preserving Th1/Th2 cytokine balance. Anesthesiology. 2007;106:499–506.
Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995;82:1474–1506.
Schneemilch CE, Ittenson A, Ansorge S, Hachenberg T, Bank U. Effect of 2 anesthetic techniques on the postoperative pro-inflammatory and anti-inflammatory cytokine response and cellular immune function to minor surgery. J Clin Anesth. 2005;17:517–527.
Crozier TA, Müller JE, Quittkat D, Sydow M, Wuttke W, Kettler D. Effect of anaesthesia on the cytokine responses to abdominal surgery. Br J Anaesth. 1994;72:280–285.
Pirttikangas CO, Salo M, Mansikka M, Grönroos J, Pulkki K, Peltola O. The influence of anaesthetic technique upon the immune response to hysterectomy. A comparison of propofol infusion and isoflurane. Anaesthesia. 1995;50:1056–1061.
Kotani N, Hashimoto H, Sessler DI, Kikuchi A, Suzuki A, Takahashi S, Muraoka M, Matsuki A. Intraoperative modulation of alveolar macrophage function during isoflurane and propofol anesthesia. Anesthesiology. 1998;89:1125–1132.
Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, Shingu K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59:954–959.
Author information
Authors and Affiliations
Additional information
This review article was invited by the Editorial Board members of the Journal of Anesthesia and was peer-reviewed as were the other articles in this journal.
About this article
Cite this article
Kurosawa, S., Kato, M. Anesthetics, immune cells, and immune responses. J Anesth 22, 263–277 (2008). https://doi.org/10.1007/s00540-008-0626-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-008-0626-2