Abstract
Background
Considerable progress has been made in our understanding of the biological basis for cancer therapy-induced mucosal barrier injury (mucositis). The last formal review of the subject by MASCC/ISOO was published in 2007; consequently, an update is timely.
Methods
Panel members reviewed the biomedical literature on mucositis pathobiology published between January 2005 and December 2011.
Results
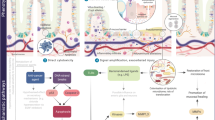
Recent research has provided data on the contribution of tissue structure changes, inflammation and microbiome changes to the development of mucositis. Additional research has focused on targeted therapy-induced toxicity, toxicity clustering and the investigation of genetic polymorphisms in toxicity prediction. This review paper summarizes the recent evidence on these aspects of mucositis pathobiology.
Conclusion
The ultimate goal of mucositis researchers is to identify the most appropriate targets for therapeutic interventions and to be able to predict toxicity risk and personalize interventions to genetically suitable patients. Continuing research efforts are needed to further our understanding of mucositis pathobiology and the pharmacogenomics of toxicity.
Similar content being viewed by others
References
Elting L et al (2003) The burdens of cancer therapy: clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98:1531–1539
Sonis S et al (2004) Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100:1995–2025
Capp A et al (2009) Is there more than one proctitis syndrome? A revisitation using data from the TROG 96.01 trial. Radiother Oncol 90:400–407
Keefe D et al (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Aprile G et al (2008) Application of distance matrices to define associations between acute toxicities in colorectal cancer patients receiving chemotherapy. Cancer 112:284–292
Murphy B (2007) Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol 5:13–21
Sonis S (2004) The pathobiology of mucositis. Nat Rev Cancer 4:277–284
Sonis S et al (2002) The gene expression sequence of radiated mucosa in an animal mucositis model. Cell Prolif 35:s92–s102
Sonis S et al (1990) An animal model for mucositis induced by cancer chemotherapy. Oral Surg, Oral Med, Oral Pathol 69:437–443
Paris F et al (2001) Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293:293–297
Logan R et al (2009) Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 63:239–251
Sonis S et al (2000) Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol 36:373–381
Yeoh A et al (2005) Nuclear factor κB (NFκB) and cyclooxygenase-2 (COX-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int J Radiat Oncol Biol Phys 63:1295–1303
Manzano M et al (2007) Intestinal toxicity induced by 5-fluorouracil in pigs: a new preclinical model. Chemotherapy 53:344–355
Stringer A et al (2009) Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90:489–499
Stringer A et al (2009) Chemotherapy-induced changes to microflora: evidence and implications of change. Curr Drug Metab 10:79–83
Stringer A et al (2007) Chemotherapy-induced diarrhea is associated with changes in the luminal environment in the DA rat. Exp Biol Med 232:96–106
Al-Dasooqi N et al (2011) Irinotecan-induced alterations in intestinal cell kinetics and extracellular matrix component expression in the dark agouti rat. Int J Exp Pathol 92:357–365
Al-Dasooqi N et al (2010) Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the DA rat. Exp Biol Med 235:1244–1256
Anthony L et al (2007) New thoughts on the pathobiology of regimen-related mucosal injury. Support Care Cancer 14:516–518
Hannum Y (1997) Apoptosis and the dilemma of cancer chemotherapy. Blood 89:1845–1853
Kerr J, Winterford C, Harmon B (1994) Apoptosis: its significant in cancer and cancer therapy. Cancer 73:2013–2026
Gibson R et al (2005) Relationship between dose of methotrexate, apoptosis, p53/p21 expression and intestinal crypt proliferation in the rat. Clin Exp Med 4:188–195
Bowen J et al (2005) Cytotoxic chemotherapy up-regulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56–62
Keefe D (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47:632–637
Sonis S et al (1992) Effect of epidermal gorwth factor on ulcerative mucositis in hamsters that receive chemotherapy. Oral Surg, Oral Med, Oral Pathol 74:749–755
Gibson R et al (2007) Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy 53:360–369
Gibson R et al (2006) Apoptosis occurs early in the basal layer of the oral mucosa following cancer chemotherapy. Asia Pac J Clin Oncol 2:39–49
Li C et al (2011) The correlation between the severity of radiotherapy-induced glossitis and endothelial cell injury in local tissues in a rat model. Med Oral Pathol Oral Cir Bucal 16:e711–e715
Carneiro-Filho B et al (2004) Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci 49:65–72
Chen P et al (2011) Role of AMP-18 in oral mucositis. Oral Oncol 47:831–839
Huang T et al (2009) Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem Biophys Res Commun 389:634–639
Meredith J, Fazeli B, Schwartz M (1993) The extracellular matrix as a cell survival factor. Mol Biol Cell 4:953–961
Afshar S, Phelan K, O'Donnell C, Bragdon C, Castro D, Sonis S (2002) A new in vivo model for the study of mucosal disease. International Association for Dental Research Meeting Abstract 16220
Phelan S, Afshar S, O'Donnell C, Bragdon C, Castro D, Shklar G, Sonis S (2002) A mucosal graft model to evaluate radiation induced injury. International Association for Dental Research Meeting Abstract 0221
Beutheu Youmba S et al (2012) Methotrexate modulates tight junctions through NFkB, MEK, and JNK pathways. J Pediatric Gastroenterol Nutr 54:463–470
Hamada K et al (2010) Zonula Occluden-1 alterations and enhances intestinal permeability in methotrexate-treated rats. Cancer Chemother Pharmacol 66:1031–1038
Al-Sadi R et al (2010) IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am J Pathol 177:2310–2322
Al-Sadi R et al (2008) Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 180:5653–5661
Ma T et al (2004) TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 286:G367–G376
Han X, Fink M, Delude R (2003) Proinflammatory cytokines cause NO*-dependent and -independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock 19:229–237
Melichar B et al (2005) Intestinal permeability in the assessment of intestinal toxicity of cytotoxic agents. Chemotherapy 51:336–338
Logan R et al (2008) Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 62:33–41
Sonis S (2002) The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit Rev Oral BiolMed 13:380–389
Sonis S (2004) A biological approach to mucositis. J Support Oncol 2:21–32
Sonis S et al (2004) The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol 40:170–176
Logan R et al (2007) Nuclear factor-kB (NFkB) and cyclooxygenase-2 expression in the oral mucosa following cancer chemotherapy. Oral Oncol 43:395–401
Haagen J et al (2009) Effect of selective inhibitors of inflammation on oral mucositis: preclinical studies. Radiother Oncol 92:472–476
Ong Z et al (2010) Pro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositis. Radiat Oncol 16:22
Logan R et al (2007) The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 33:448–460
Logan R et al (2008) Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 7:1139–1145
Lima V et al (2005) Effects of tumour necrosis factor-alpha inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur J Oral Sci 113:210–217
Melo M et al (2008) Role of cytokines (TNF-alpha, IL-1beta and KC) in the pathogenesis of CPT-11-induced intestinal mucositis in mice: effect of pentoxifylline and thalidomide. Cancer Chemother Pharmacol 61:775–784
Fiochhi C (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182–205
de Koning B et al (2006) Contributions of mucosal immune cells to methotrexate-induced mucositis. Int Immunol 18:941–949
Bultzingslowen I et al (2006) Growth factors and cytokines in the prevention and treatment of oral and gastrointestinal mucositis. Support Care Cancer 14:519–527
Gibson R et al (2002) Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 47:2751–2757
Antin J et al (2002) A phase I/II double-blind, placebo-controlled study of recombinant human interleukin-11 for mucositis and acute GVHD prevention in allogeneic stem cell transplantation. Bone Marrow Transplant 29:373–377
Zhao J et al (2004) Oral RDP58 allows CPT-11 dose intensification for enhanced tumor response by decreasing gastrointestinal toxicity. Clin Cancer Res 10:2851–2859
Frei E et al (1965) The nature and control of infections in patients with acute leukemia. Cancer Res 25:1511–1515
Dreizen S, Bodey G, Brown L (1974) Opportunistic gram-negative bacillary infections in leukemia. Oral manifestations during myelosuppression. Post Med 55:133–139
Stringer A et al (2008) Faecal microflora and β-glucuronidase expression are altered in an irinotecan-induced diarrhoea model in rats. Cancer Biol Ther 7:1919–1925
Stringer A et al (2009) Gastrointestinal microflora and mucins play a role in the development of 5-fluorouracil-induced gastrointestinal mucositis in rats. Exp Biol Med 234:430–441
Shao Z et al (2011) Effects of intensity-modulated radiotherapy on human oral microflora. J Radiat Res 52:834–839
Napenas J et al (2010) Molecular methodology to assess the impact of cancer chemotherapy on the oral bacterial flora: a pilot study. Oral Surg, Oral Med, Oral Pathol, Oral Radiol Endodentics 109:554–560
van Vliet M et al. (2010) The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog 6(5): e1000879
Martin M, van Saene H (1992) The role of oral microorganisms in cancer therapy. Curr Opin Dent 2:81–84
Bochud P et al (1994) Bacteremia due to viridans streptococcus in neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis 18:25–31
Ruescher T et al (1998) The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 82:2275–2281
Soga Y et al (2011) Bacterial substitution of coagulase-negative staphylococci for streptococci on the oral mucosa after hematopoietic cell transplantation. Support Care Cancer 19:995–1000
van der Velden W, Donnelly J, Blijlevens N (2012) Lymphocyte subsets, granulocyte-colony-stimulating factor responsiveness and post-stem cell transplantation infections: mucositis is the underestimated confounder? Cytotherapy 14:381–383
Sonis S (2009) Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 45:1015–1020
Barasch A et al (2006) Antimicrobials, mucosal coating agents, anesthetics, analgesics, and nutritional supplements for alimentary tract mucositis. Support Care Cancer 14:528–532
Blijlevens N, Donnelly J, DePauw B (2000) Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transplant 25:1269–1278
Prisciandaro L et al (2011) Probiotic factors partially improve parameters of 5-fluorouracil-induced intestinal mucositis in rats. Cancer Biol Ther 11:671–677
Gibson R et al. (2013) Systematic review of agents for the management of gastrointestinal mucositis in cancer patients. Supportive Care Cancer 21(1):313-26
Blijlevens N, Donnelly J (2011) Mucosal barrier injury and infections. In: Safdar A (ed) Principles and practice of cancer infectious diseases, current clinical oncology. Springer Science, New York
Wisplinghoff H et al (2003) Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis 36:1103–1110
Keefe D, Bateman E (2011) Tumor control versus adverse events with targeted anticancer therapies. Nat Rev Clin Oncol 9:98–109
Sonis S et al (2010) Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer 116:210–215
Toi M et al (2009) Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer 101(10):1676–1682
Sawaki M et al (2004) Efficacy and safety of trastuzumab as a single agent in heavily pretreated patients with HER-2/neu-overexpressing metastatic breast cancer. Tumori 90(1):40–43
Keefe D, Gibson R (2007) Mucosal injury from targeted anticancer therapy. Support Care Cancer 15:483–490
Burris H (2004) Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist 9:10–15
Geyer CE et al (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355(26):2733–2743
Al-Dasooqi N et al (2008) Trastuzumab induces gastrointestinal side effects in HER2-overexpressing breast cancer patients. Investig New Drugs 27:173–178
Fountzilas G et al (2001) Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol 12:1545–1551
Bartsch R et al (2007) Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol 25:3853–3858
Ruiz M et al (2008) Phase-II study of weekly schedule of trastuzumab, paclitaxel, and carboplatin followed by a week off every 28 days for HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 62:1085–1090
Bowen J et al (2012) Development of a rat model of oral small molecule receptor tyrosine kinase inhibitor-induced diarrhoea. Cancer Biol Ther 13(13):1269-75
Peterson D, Keefe D, Sonis S (2012) New frontiers in mucositis. ASCO Educational Book. American Society of Clinical Oncology, Alexandria, pp. 545–61
Keefe D (1998) The effect of cytotoxic chemotherapy on the mucosa of the small intestine. Department of Medicine University of Adelaide, Adelaide
Pico J, Avila-Garavito A, Naccache P (1998) Mucositis: its occurence, consequences and treatment in the oncology setting. The Oncologist volume 3:p446–451
Sloan J et al (2002) Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol 20:1491–1498
Pratesi N et al (2011) Association between single nucleotide polymorphisms in XRCC1 and RAD51 genes and clinical radiosensitivity in head and neck cancer. Radiother Oncol 99:356–362
West C, Dunning A, Rosenstein (2012) Genome-wide association studies and prediction of normal tissue toxicity. Sem Radiat Oncol 22:91–99
Thomas F et al (2011) Methylenetetrahydrofolate reductase genetic polymorphisms and toxicity to 5-FU-based chemoradiation in rectal cancer. Br J Cancer 105:1654–1662
Werbrouck J et al (2009) Acute normal tissue reactions in head-and-neck cancer patients treated with IMRT: influence of dose and association with genetic polymorphisms in DNA DSB repair genes. Int J Radiat Oncol Biol Phys 73:1187–1195
Schwab M et al (2008) Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol 26:2131–2138
Cho H et al (2010) Glutathione-S-transferase genotypes influence the risk of chemotherapy-related toxicities and prognosis in Korean patients with diffuse large B-cell lymphoma. Cancer CGenet Cytogenet 198:40–46
Hahn T et al (2010) A deletion polymorphism in glutathione-S-transferase mu (GSTM1) and/or theta (GSTT1) is associated with an increased risk of toxicity after autologous blood and marrow transplantation. Biol Blood Marrow Transplant 16:801–808
Alterovitz G et al (2011) Personalized medicine for mucositis: bayesian networks identify unique gene clusters which predict the response to gamma-D-glutamyl-L-tryptophan (SCV-07) for the attenuation of chemoradiation-induced oral mucositis. Oral Oncol 47:951–955
Roseth A (2003) Determination of faecal calprotectin, a novel marker of organic gastrointestinal disorders. Dig Liver Dis 35:607–609
Lutgens L et al. (2005) Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer 103(1):191–9
Xanthinaki A et al (2008) Apoptosis and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary report. Support Care Cancer 16:1025–1033
Conflict of interest
This project was carried out as part of the MASCC/ISOO Mucositis Guidelines Update, which was supported by BioAlliance Pharma and Helsinn Healthcare, NA. No industry representatives participated in the development of this manuscript in any way.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Dasooqi, N., Sonis, S.T., Bowen, J.M. et al. Emerging evidence on the pathobiology of mucositis. Support Care Cancer 21, 2075–2083 (2013). https://doi.org/10.1007/s00520-013-1810-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1810-y