Abstract
Introduction
Late-stage ovarian cancer patient’s survival depends on complete cytoreduction and chemotherapy. Complete cytoreduction is more often achieved in institutions with a case volume of >20 cases per year. The Integrated care program Ovar (IgV Ovar) was founded in 2005 and started recruiting in 2006 with 21 health insurances and six expert centers of ovarian cancer treatment as a quality initiative. Results of the pilot and outcomes of patients of three participating centers will be presented here.
Methods
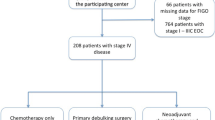
Data of 1038 patients with ovarian cancer were collected. Adjuvant patients (n = 505) stage FIGO IIB-IV (n = 307) were analyzed for cytoreduction and survival. FIGO IIIC patients were analyzed separately.
Results
Median follow-up was 32.7 months. Progression-free survival (PFS) was 23.1 months and overall survival (OS) was 53.6 months for stage IIB-IV. Patients with FIGO IIIC were completely cytoreduced in 48 %. PFS was 21, 29 months if completely cytoreduced. OS was 47.4, 64.9 months if completely cytoreduced.
Discussion
Although the IgV Ovar Rhineland proved to have some structural problems with recruitment and prospective data collection, cytoreduction rates and outcome of patients prove treatment of patients in expert centers is superior to the national and international mean. Therefore, a new quality initiative will be started to bring more awareness to women and to their gynecologists and general practitioners of just how important a good referral strategy is.
Similar content being viewed by others
Introduction
With 75 % ovarian cancer is still typically diagnosed at late stages, at this point, peritoneal carcinosis has most often spread to all quarters of the abdomen and/or to the pleural surfaces (FIGO IIIC or IVA). These women have a poor prognosis despite all efforts and have a 5-year survival rate of <25 %. Over three quarters of these patients present with serous papillary histology (Heintz et al. 2006).
Therapy of the disease is a combination of surgery and chemotherapy. Agents used for chemotherapy have been platinum, usually carboplatin, and a taxane, most often paclitaxel. Despite a few changes in regimen or (internationally) in sequence and route of application from intravenous to adding intraperitoneal, this has not changed for more than a decade (du Bois et al. 2003, 2010; Armstrong et al. 2006; Polcher et al. 2009; Pignata et al. 2014).
Prognostic factors are age, performance status, stage, histologic type, grading, pN1 status and the completeness of cytoreduction (Chi et al. 2001). Whereas the first six parameters are inherent, cytoreduction is influenced not only by the sites of the metastatic peritoneal tumor but greatly by the abilities of the individual surgeon and by the infrastructure available at the hospital (Kuhn et al. 2001; Crawford et al. 2005; Chi et al. 2006).
Researchers, mainly from the USA, but also from Germany, have shown treatment in hospitals with an interdisciplinary approach of the gynecologic oncologist as a surgeon with visceral surgeons and urologists improves the outcome of patients with ovarian cancer (Wimberger et al. 2007; Bristow et al. 2009). Equally important to the structure and case volume of the hospital is the experience and case volume of the operating gynecological surgeon (du Bois et al. 2009; Bristow et al. 2014).
Unfortunately, besides these well-published and researched facts, in Germany treatment of patients with late-stage ovarian cancer is not restricted to specialized centers. Basically, any surgeon’s belief in his or her own abilities qualifies for the undertaking of treatment of these patients, in some cases producing surgical outcomes of low quality with impaired survival of the patient.
In 2005, by initiative of the public health insurances, specialized gynecologic oncologists from six centers for treatment of ovarian cancer in the Rhineland came together with 21 health insurers forming the idea of an integrated care program (IgV) for patients with ovarian cancer.
The program was implemented to increase the amount of patients being treated in hospitals with high quality of surgical performance and in hospitals with adequate infrastructure, therefore increasing the rate of successfully completely cytoreduced patients.
Results from the “Integrated care in ovarian cancer (“IgV Ovar”) Rhineland” and outcomes of patients from three participating hospitals will be presented here.
Methods
Criteria of quality were developed with international specialists of the field. They were laid down in a contract with the health insurance companies for hospitals wanting to take part in the initiative. These included structural criteria of the clinic as a whole for measurement of the interdisciplinary work with visceral surgeons and urologists, structural quality criteria for the gynecologic department for insuring operations by board-certified gynecologic surgeons, and number of carried out operations on patients with ovarian cancer in the last years per department and per surgeon and for chemotherapy application.
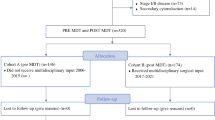
After diagnosis of ovarian cancer, each patient insured with any of the participating health insurances was asked whether she would like to participate in this quality initiative. If the patient was interested and registered per informed consent, the referring gynecologist was asked whether he/she was willing to cooperate, integrating the practice into the after-treatment clinical follow-up, with the patient presenting at the clinic and the referring gynecologist one time over the other.
Similar to other integrated programs (e.g., DMP Mamma), the referring gynecologist was asked to register with the initiative and would receive a fee for completing some follow-up forms on seeing the patients. Patients were to answer some Quality of life (QoL) questionnaires on presenting for clinical follow-up. All generated data were to be sent to the individual health insurance of the patient per fax.
To prove the idea, treatment of ovarian cancer patients in centers of experience would lead to better outcome of these patients, and to prove the principal idea of a centralized care for these patients, three of the participating hospital agreed to pool their data for treatment of patients with all stages of ovarian cancer in their institutions. To analyze quality of treatment, focus was laid on adjuvant treated patients with advanced stages (FIGO IIB–IV) since these patients represent a homogenously treated group. More analysis was done for FIGO IIIC patients, for these are presenting with surgically challenging conditions.
Data of all patients, treated in the Department of Obstetrics and Gynecology of the Helios Klinikum in Krefeld, the Evangelisches Krankenhaus in Düsseldorf and the University Hospital in Bonn were collected retrospectively. Since all hospitals are certified by the Deutsche Krebsgesellschaft (DKG) as “Gynäkologische Krebszentren”, significant parts of the follow-up were available, and missing data were completed.
Statistical analysis was performed using the IBM program SPSS 22, IBM, Ehningen, Germany.
Results
Data of all patients surgically treated with ovarian cancer in the three hospitals between 2006 and 2012, 1038 patients in total, were collected (see Table 1). Out of 505 patients with adjuvant treatment, 307 patients were treated with advanced disease (IIB–IV) used for this analysis (see Table 2).
The median age was 63.4 years (range 25–100 years), 79 % presenting with FIGO III disease. ASA classification (grading of patients for surgical procedures of the American Society of Anesthesiologists) was used as surrogate marker for performance status and was ASA 2 in 61 % and ASA 3 in 24 % of cases. Twenty-four percentage of patients presented with Grade 3 tumors, and 38 % had lymph node involvement. Fifty percentage of patients underwent systematic lymphonodectomy, in which 9.5 % bulky nodes were removed. Median number of removed nodes was 38 (95 % CI 7–138). Median operation time was 290 min (95 % CI 29–695 min).
Fifty percentage of patients were cytoreduced to no residual tumor, and 28 % had residual tumor ≤1 cm (Table 2).
Median follow-up was 32.7 months (95 % CI 0.5–100 months). Progression-free survival (PFS) was 23.1 months (95 % CI 20.0–26.1 months) (Fig. 1) and overall survival (OS) was 53.6 months (95 % CI 41.6–65.5 months) in patients with stage IIB–IV (Fig. 2). To this group of adjuvant patients, clinic A contributed 73 out of 156 patients with stage FIGO IIB–IV operated on during this time, clinic B 137 out of 156 and clinic C 97 out of 107. All three centers are therefore very much equal in their hospital volume for advanced ovarian cancer surgery, and survival time did not differ between the clinics (OS p = 0.963; PFS p = 0.327).
Patients with FIGO IIIC (n = 198) were completely cytoreduced in 92 cases (46.46 %), and 61 patients (30.81 %) were cytoreduced to ≤1 cm (Table 3). PFS was 21 months (95 % CI 18–24 months), 28 months if completely cytoreduced (95 % CI 16.2–41.7 months; p = 0.001), 18.9 months (95 % CI 15.2–22.5 months) if a residual tumor of ≤1 cm, 16.2 months (95 % CI 13.1–19.4 months) if tumor residuals of >1 cm remained (Fig. 3; Table 3).
OS was 47.4 months (95 % CI 38.8–55.9 months) and 64.9 months (95 % CI 63.6–66.2 months; p = 0.001) if completely cytoreduced, 34.1 months (95 % CI 28.9–39.2 months) if a residual tumor of ≤1 cm, and 34.8 months (20.1–49.4 months) if tumor residuals of >1 cm remained (Fig. 4; Table 4).
Discussion
Pooled data analysis proved the selection criteria for participating hospitals successfully insured the desired improvement of quality of treatment for the patient.
In 2000, 2001, 2004 and 2009, the Arbeitsgemeinschaft für Gynäkologische Onkologie Ovar (AGO Ovar) published surveys on German hospitals for quality of treatment, using postoperative status of cytoreduction as the most important indicator (Hilpert F 2010). Over the surveyed years, the ratio of patients with FIGO II–IV with complete cytoreduction was improved from initially less than 25 % to about 41 % in the last survey. The participating IgV hospitals achieved a rate for complete cytoreduction of 51 % (Fig. 5).
Cytoreduction: comparison QS-Ovar versus IgV hospitals. Modified after QS-Ovar, Hilpert et al. (2010)
The well-established Munich tumor register reports a 32 % rate of complete tumor resection for patients with stage FIGO IIIC disease (Schmalfeldt et al. 2014). The participating IgV hospitals achieved a rate of complete cytoreduction of 47 % in the same collective.
Data from FIGO IIIC patients show the importance of complete tumor resection (Figs. 3, 4). Residual tumor of any size will compromise the survival of the patient, since there is no statistical difference between gross residual tumor and tumor lesions under or over 1 cm (OS 34.1 vs. 34.8 months, n.s.). This result is to be expected and ties in with most published data (Chang and Bristow 2012; Keyver-Paik et al. 2013). The same effect of any residual tumor was seen for all other stages in the performed analysis outside FIGO IIIC (data not shown).
With high rates of complete cytoreduction, PFS and OS were 23.1 and 53.6 months, respectively, for patients with FIGO IIB–IV comparing to PFS and OS survival rates in literature of 18.2 and 44.1 months in a German collective of patients on chemotherapy trials (du Bois et al. 2009). International trial results, e.g., ICON7, show a PFS of 17.5 in the control and 19.9 in the research arm (+bevacizumab) and an OS of 30.3 versus 39.7 months for stages I–II high risk and IIB–IV (Perren et al. 2011; E Pujade-Lauraine 2014). In patients with stage FIGO IIIC, the three IgV hospitals achieved a PFS of 21 months for all patients and 29 months when no residual tumor was present. Overall survival was 47.4 months for all and 64.9 when no residual tumor was present. In ICON7, stage FIGO IIIC patients achieved a PFS of 17.5 versus 19.9 and an OS of 58.6 versus 58.0 months. Although retrospective data, the results of the three IgV hospitals are representing an excellent outcome for patients treated in these hospitals especially considering the data comes from a non-trial patient collective.
In 2009, du Bois et al. published a meta-analysis of surgical outcome comparing operations by board-certified gynecologic oncologists to other gynecologic surgeons or surgeons of other disciplines, e.g., visceral surgeons (du Bois et al. 2009). The data clearly was in favor of the specialized gynecologic oncologists, showing the knowledge of the disease and the acknowledgement of cytoreduction as the one most important and influenceable factor in ovarian cancer is the key to a successful operation.
Other published data are showing, when comparing surgical effort in experienced centers to less-experienced centers, less-experienced centers will significantly underperform (all p < 0.0001) in systematic lymphonodectomy, bowel surgery and specifically in peritoneal stripping. In this analysis, it is most discomforting to see, even basic measures, as hysterectomy and bilateral adnexectomy and omentectomy are significantly less frequently carried out in less-experienced centers (p < 0.0001) (Wimberger et al. 2007).
A data analysis of 20,600 patients in the USA, published in 2010, also proved a statistical correlation between case volume for FIGO IIIC and IV patients and survival (Bristow et al. 2010). A case volume of >20 cases per hospital showed a better overall survival for these patients. A later analysis of the same author of almost 12,000 patients defines high-volume hospitals as hospitals with >20 cases, and high-volume physicians as physicians with >10 cases (Bristow et al. 2014). Patients treated in high-volume hospitals by high-volume physicians have a statistically significant better survival than patients treated by surgeons with less cases in smaller hospital units. Any other combination is also less favorable than the high-volume combination.
These results were published well after the contracting for the IgV Ovar in 2006, but precisely underline the correctness of the quality strategy applied by the network.
Cost effectiveness was no parameter in the planning of the IgV Ovar but data from literature suggest a referral strategy to more experienced centers is also cost-effective. An American analysis from 2007 showed although treatment in experienced centers may be more expensive initially (39,957$ vs. 50,652$), when adjusted for QoL and survival time, experienced centers were the more cost-effective healthcare strategy (17,149$ vs. 9893$) (Bristow et al. 2007).
This analysis of outcome proves the hypothesis of bettering the treatment of patients in specialized centers, and it is probably also a cost-effective strategy, albeit there were also some problems in the pilot model of integrated care.
Data collection was not done to the standard aimed for at the beginning of this pilot. Quality of data on QoL proved insufficient for a comprehensive analysis.
After some thorough investigation into reasons given for the reported shortfalls in data collection and recruitment, the cardinal points are:
Referring gynecologists were more reserved to the program than expected: partly, since participation of their patients in this program meant more documentation on an occupation already filled with documentation on a daily basis, and partly because of a general distrust toward a data collection being forwarded to the participating health insurers.
On the side of the hospitals’ clinicians, identifying eligible patients for the program was also some obstacle to overcome because of the myriad of health insurances, in some cases with a very local structure, in the German health system. Only patients with the participating insurances were to be included in the program, and in some cases, although one local branch of an insurance company may take part, another local branch of the same insurance might not participate. This made it difficult to oversee for the clinician to which patient to offer the program without permanently carrying a list of the insurances. No private insurance did participate in the program.
A negative attitude toward more documentation was also one of the stumbling blocks for hospital clinicians. All hospitals are certified “Gynäkologische Krebszentren” and documentation and follow-up are already done. Hospitals employ staff for documentation of patients with gynecological cancer and their treatment and follow-up, but with no common electronic platform, and using an overcome system of paper and fax, double documentation of the same patient was necessary.
There was no regular central controlling mechanism until the end of the project in 2015. Therefore, regular surveillance on data collection and data quality was not established. The decentralized mode of data collection in the different clinics, private practice and health insurances helped to cover up the lack of complete documentation for some extended time.
While the contract was very specific on the quality assurance of the hospitals structure for treatment for patients, it was lacking to specify the quality of structure for documentation. It would have been advisable to install a case manager for patients on the program in every hospital.
Ovarian cancer still is a disease with a poor prognosis. Surgical outcome with complete cytoreduction is the one most important prognostic factor for the survival of a patient. Too many patients in Germany today are still not referred to centers of experience, where an interdisciplinary setup and an experienced and well-educated gynecological surgeon will see to the success of the cytoreduction. A suboptimal referral strategy still costs life and life-time even today in Germany.
The quality initiative “IgV Ovar” in the Rhineland was a project set up by some health insurances and initiating expert centers of the region and under the consultation of international experts in this field. IgV Ovar did successfully prove, treatment in expert centers leads to a better outcome of patients. Therefore, although not all expectations were met, a new initiative will be started soon based on the concluded pilot.
Avoiding double documentation and excessive administrative detail, we will approach hospitals to come together with the assistance of the health insurance companies to voluntarily publish their quality indicators of case volume and outcomes in a benchmarking system. In return, we will initiate an awareness campaign for patients, gynecologists and general practitioners publishing the benchmark and emphasizing the importance to be treated in expert centers when ovarian cancer is diagnosed.
References
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354(1):34–43
Bristow RE, Santillan A, Diaz-Montes TP, Gardner GJ, Giuntoli RL 2nd, Meisner BC, Frick KD, Armstrong DK (2007) Centralization of care for patients with advanced-stage ovarian cancer: a cost-effectiveness analysis. Cancer 109(8):1513–1522
Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK (2009) Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol 115(3):334–338
Bristow RE, Palis BE, Chi DS, Cliby WA (2010) The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol 118(3):262–267
Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H (2014) High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol 132(2):403–410
Chang SJ, Bristow RE (2012) Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: redefining ‘optimal’ residual disease. Gynecol Oncol 125(2):483–492
Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, Hoskins WJ (2001) Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol 82(3):532–537
Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR (2006) What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 103(2):559–564
Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB (2005) Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC-1 Trial. J Clin Oncol 23(34):8802–8811
du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, Bauknecht T, Richter B, Warm M, Schroder W, Olbricht S, Nitz U, Jackisch C, Emons G, Wagner U, Kuhn W, Pfisterer J (2003) A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 95(17):1320–1329
du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115(6):1234–1244
du Bois A, Herrstedt J, Hardy-Bessard AC, Muller HH, Harter P, Kristensen G, Joly F, Huober J, Avall-Lundqvist E, Weber B, Kurzeder C, Jelic S, Pujade-Lauraine E, Burges A, Pfisterer J, Gropp M, Staehle A, Wimberger P, Jackisch C, Sehouli J (2010) Phase III trial of carboplatin plus paclitaxel with or without gemcitabine in first-line treatment of epithelial ovarian cancer. J Clin Oncol 28(27):4162–4169
Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U (2006) Carcinoma of the ovary. FIGO 6th Annual Report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 95(Suppl 1):S161–S192
Hilpert F, Harter P, Pfisterer J, Rochon J, Friedemann J, Lamparter C, du Bois A (2010) Eigenverantwortliche Qualitätssicherung zur Therapiequalität des Ovarialkarzinoms in Deutschland: das QS-OVAR-Projekt der Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) Kommission OVAR. Der Gynäkologe 43:1022–1027
Keyver-Paik MD, Zivanovic O, Rudlowski C, Holler T, Wolfgarten M, Kubler K, Schroder L, Mallmann MR, Polcher M, Kuhn W (2013) Interval debulking surgery in patients with Federation of Gynecology and Obstetrics (FIGO) stage IIIC and IV ovarian cancer. Onkologie 36(6):324–332
Kuhn W, Rutke S, Spathe K, Schmalfeldt B, Florack G, von Hundelshausen B, Pachyn D, Ulm K, Graeff H (2001) Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for patients with poor prognosis in International Federation of Gynecology and Obstetrics Stage IIIC ovarian carcinoma. Cancer 92(10):2585–2591
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365(26):2484–2496
Pignata S, Scambia G, Katsaros D, Gallo C, Pujade-Lauraine E, De Placido S, Bologna A, Weber B, Raspagliesi F, Panici PB, Cormio G, Sorio R, Cavazzini MG, Ferrandina G, Breda E, Murgia V, Sacco C, Cinieri S, Salutari V, Ricci C, Pisano C, Greggi S, Lauria R, Lorusso D, Marchetti C, Selvaggi L, Signoriello S, Piccirillo MC, Di Maio M, Perrone F (2014) Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 15(4):396–405
Polcher M, Mahner S, Ortmann O, Hilfrich J, Diedrich K, Breitbach GP, Hoss C, Leutner C, Braun M, Mobus V, Karbe I, Stimmler P, Rudlowski C, Schwarz J, Kuhn W (2009) Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer–a prospective multicenter phase II trial (PRIMOVAR). Oncol Rep 22(3):605–613
Pujade-Lauraine E, Oza A, Perren TJ, Swart AM, Mahner S, Gourley C, Malander S, Plante M, Beale P, Poveda A, Kaplan R, Embleton AC, Parmar M (2014) ICON7: Final overall survival results in the GCIG phase III randomised trial of bevacizumab in newly diagnosed ovarian cancer. In: Oral Presentation at the ESGO 2013
Schmalfeldt B, Burges A, Mayr D, Schubert-Fritschle G, Rottmann M, Engel J (2014) Qualität der Therapie des Ovarialkarzinoms in der Region des Tumorzentrums München. Epidemiologische Daten im Vergleich. Geburtshilfe Frauenheilkd 2014 74 - PO_Onko07_11
Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A (2007) Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 106(1):69–74
Acknowledgments
We would like to thank the participating health insurances for their partnership in the IgV Ovar. We thank Professor I. Vergote and Professor C. Marth for their consultation and input into the project. We thank Ms. C. Otto of the documentationists’ pool of the Center for Integrated Oncology (CIO) Köln Bonn and all other co-workers from Düsseldorf and Krefeld for assisting in the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Keyver-Paik, MD., Abramian, A., Domröse, C. et al. Integrated care in ovarian cancer “IgV Ovar”: results of a German pilot for higher quality in treatment of ovarian cancer. J Cancer Res Clin Oncol 142, 481–487 (2016). https://doi.org/10.1007/s00432-015-2055-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2055-6