Abstract
ATP-binding cassette (ABC) transporters are ubiquitous integral membrane proteins that facilitate the transbilayer movement of ligands. They comprise, minimally, two transmembrane domains, which impart ligand specificity, and two nucleotide-binding domains (NBDs), which power the transport cycle. Almost 25 years of biochemistry is reviewed in light of the recent structure analyses resulting in the ATP-switch model for function in which the NBDs switch between a dimeric conformation, closed around two molecules of ATP, and a nucleotide-free, dimeric ‘open’ conformation. The flexibility of this switching mechanism has evolved to provide different kinetic control for different transporters and has also been co-opted to diverse functions other than transmembrane transport.
Similar content being viewed by others
Introduction
The translocation of molecules across cellular lipid membranes is critical for most aspects of cell physiology including the uptake of nutrients and the elimination of waste products, energy generation and cell signalling. ATP-binding cassette (ABC) transporters are one of the major classes of membrane transporters found in all cell types of all species so far studied [1]. Different ABC transporters translocate different substrates, ranging from small ions to large polypeptides, and they therefore play a wide variety of physiological roles. Mutations in ABC transporter genes underlie a number of human genetic disorders, as discussed elsewhere in this issue. They are also of particular economic and medical importance as they can pump cytotoxic molecules from cells conferring resistance to antibiotics, herbicides and chemotherapeutic drugs.
ABC transporters utilise the energy released by ATP binding, ATP hydrolysis and ADP-Pi release (henceforth referred to as ATP catalysis, although it is important to remember that energy is available at each of these steps) to transport substrates, vectorially, across cell membranes. All eukaryotic ABC transporters that have been characterised transport their substrate from the cytoplasm, out of the cell or into organelles, although some bacterial ABC transporters have acquired an additional extracellular protein, the periplasmic binding protein (PBP), to facilitate substrate import. Two apparently ‘typical’ mammalian ABC proteins do not appear to mediate active transport but instead have evolved to couple the conformational changes induced by ATP catalysis to regulate K+ channel activity (SUR1/ABCC8), or to open and close a chloride channel formed by the ABC “transporter” itself (CFTR/ABCC7). Finally, several ABC nucleotide-binding domains (NBDs) have evolved to couple ATP catalysis to distinct biological functions such as DNA binding/chromosome organisation (SMC proteins), DNA repair (MutS, Rad50) and mRNA export from the nucleus (Elf1p).
An understanding of how ABC transporters work to mediate directional transport of substrates across lipid membranes has been a challenge since the first sequence of a complete ABC transporter was obtained nearly 25 years ago [2]. Insights from recent biochemical, structural and genetic studies of several ABC transporters have led to the ‘ATP switch’ model of function [3].
Structure of ABC transporters
The notion that a typical ABC transporter consists of four core domains was established many years ago ([4]; Fig. 1a). These four core domains appear to form the minimal functional unit both necessary and sufficient for transport. The two transmembrane domains (TMDs) of each ABC transporter consist of multiple membrane-spanning α-helices (typically, but not always, six α-helices per domain) and form the pathway through which substrate crosses the membrane. These domains also form the substrate-binding site (or sites) which contributes to transport specificity. The two nucleotide-binding domains (NBDs) couple the energy of ATP catalysis to transport. The NBDs of all ABC transporters share extensive amino acid sequence identity, and a number of characteristic motifs define this superfamily of proteins and distinguish them from other ATP-binding proteins [4].
Structure of ABC transporters. a Schematic representation, illustrated for P-glycoprotein [17]. The NBDs are coloured in cyan and gold and shown in the closed dimer conformation, with ATP in stick form, and coloured elementally, at the interface. The TMDs, shown in blue and red, form an aqueous chamber in the membrane (the plane of which is indicated by grey rectangles). b The closed NBD dimer viewed from above, as if looking down through the membrane and TMDs (which are hidden for clarity). The two ATP molecules and two magnesium ions (black spheres) occupy the two nucleotide-binding pockets at the interface between the NBDs. c Schematic representation of NBD1 (gold) viewed from NBD2 (which is hidden for clarity). The ATP and magnesium ion coordinated predominantly by NBD1 are shown in elemental colours. The residues and motifs coordinating the MgATP are shown in stick form and coloured as follows: core domain; stacking aromatic (Y401, light purple), Walker A motif (427-GSGCGKST-435, cyan), Walker B motif (551-ILLLDEAT-558, yellow), Q-loop glutamine (Q475, dark purple), H-loop histidine (H587, green). The D-loop aspartate (D562, red) of the core domain forms an H-bond with the Walker A of NBD2 and is located at the amino terminal end of helix 6 which may be distorted during ATP hydrolysis to store energy to force ADP release from the open NBD dimer conformation [40]. The ABC signature motif (531-LSGGQ-535, blue) of the helical domain contacts the MgATP (coloured grey) that is otherwise coordinated by NBD2
The four domains of an ABC transporter are sometimes encoded as separate polypeptides and sometimes fused into multidomain proteins [4]. For any given transporter, the two NBDs are often closely related to each other (indeed, they are sometimes identical), as are the two transmembrane domains. Thus, it has long been predicted that ABC transporters are pseudodimers, with each half of the dimer consisting of one TMD and one NBD (Fig. 1a). This has now been confirmed by structural studies [5–7].
Structural data for ABC transporters have been hard to come by. The first high-resolution X-ray structure, of an isolated NBD from the Salmonella histidine transporter (HisP; [8]), has been followed by over a dozen NBD structures. All, not unexpectedly, have a very similar tertiary fold. Some of these isolated NBDs crystallised as dimers, and, initially, several distinct dimer interfaces were observed. However, it is now clear from genetic and biochemical data that the dimer interface first observed in the Rad50 [9], LolD [10] and MutS [11, 12] structures, and subsequently in the complete BtuCD transporter [6], reflect the physiological interface. In this dimer, the two nucleotide-binding pockets are located at the interface between the monomers, with both bound nucleotides coordinated by the conserved amino acids from both NBDs (Fig. 1b,c). Thus, it is appropriate to consider an ABC transporter (at least in the closed dimer form—see below) having two ATP-binding pockets, with both NBDs contributing to each pocket, rather than each NBD having a separate ATP-binding site. Throughout this review, it is sometimes convenient to refer to the activity of an individual NBD. In such cases, we mean the catalytic site to which the said NBD contributes the core subdomain including the Walker A and B motifs.
The TMDs of ABC transporters have, in contrast to the NBDs, been relatively refractory to structural analysis. Although it has long been predicted from primary sequence data that the TMDs each consist of multiple membrane-spanning α-helices, it was only with the first X-ray structure, for a bacterial lipid transporter MsbA, that this was formally confirmed [7]. Low-to-medium resolution structures of the mammalian multidrug resistance P-glycoprotein (P-gp; ABCB1), obtained by electron microscopy of single particles [13] and electron cryo-microscopy of 2-D crystals [5, 14], showed that the TMDs form an enclosed aqueous chamber/pore in the membrane which, in the basal state, appears open at the extracellular face but closed intracellularly, with the two NBDs exposed as cytoplasmic lobes (Fig. 1). Subsequent low resolution electron microscopy confirmed a similar organisation for other ABC transporters, and this was elegantly illustrated at high resolution for the Escherichia coli vitamin B12 transporter BtuCD ([6]; Fig. 2). The overall architecture of ABC transporters is distinct from that of other ATP-dependent transporters such as the P-type ATPases (Ca2+ pump) or F1-F0 ATPase in which the membrane-spanning α-helices are more closely packed and the ATP-binding domains are separated by some distance from the membrane domains. It is interesting to note that the structure of the lipid A transporter, MsbA, of E. coli suggested a very different architecture ([7]; Fig. 2a). This structure is hard to reconcile with biochemical and other data, and structures of the same protein from other species differ yet again, only one of which is compatible with the domain organisation of BtuCD ([6, 15, 16]; Fig. 2). Although it has been suggested that these very different structures may reflect different intermediate conformational states in the transport cycle, this is not consistent with biochemical and other data, and it seems more likely that in crystallisation, the four domains of MsbA may readily reorient inappropriately with respect to each other and that some of these structures may not reflect physiological configurations [17].
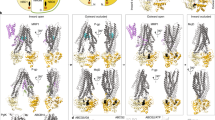
The X-ray structures of “full-length” ABC transporters. a The lipid A transporter MsbA from E. coli. The “repaired model” [83] is shown which was derived from the α-carbon chain of the crystal structure resolved to 4.5 Å [7]. b MsbA from Vibrio cholera resolved to 3.8 Å (pdb file 1PF4; [15]). c MsbA from Salmonella typhimurium crystallised with ADP.Vi and lipopolysaccharide and resolved to 4.2 Å (pdb file 1Z2R; [16]). d The vitamin B12 transporter BtuCD from E. coli resolved to 3.2 Å (pdb file 1L7V; [6]). TMDs are coloured dark grey and dark blue, while the nucleotide-binding domains are coloured light grey and cyan. All structures are viewed from a similar perspective centred on the rotational symmetry plane of the two NBDs. MsbA is a homodimer of a polypeptide comprising one TMD and one NBD. BtuCD is comprised of four polypeptide chains, homodimeric TMDs and homodimeric NBDs. The TMDs of BtuCD are non-homologous with the TMDs of MsbA but all of the NBDs are homologous. The ABC signature motif of the grey NBD is shown as spheres and coloured green. The Walker A motif of the cyan NBD of each transporter (which is expected to contact the same molecule of ATP as the ABC signature of the grey NBD) is shown as spheres and coloured red. Tetra-vanadate, ADP and LPS where present are shown in stick form. Images were generated using MacPyMOL (Delano Scientific)
The structural similarities between NBDs imply that they share a common evolutionary origin. In contrast, the TMDs of different sub-families of ABC transporters, often consisting of varying numbers of transmembrane α-helices, share little sequence identity. The two TMDs (BtuCD and MsbA; [6, 7, 15, 16]) for which high-resolution structures are available are from different sub-families and adopt different folds which cannot be modelled on one another [14]. It therefore seems most likely that all NBDs share a common origin and mechanism for coupling ATP catalysis to transport (and other biological processes) but that the NBDs are coupled to different evolutionary families of TMDs. This potentially explains how different ABC transporters can handle extremely diverse substrates, ranging from small ions to large polypeptides.
Mechanisms of transport
Studies of bacterial ABC transporters first showed that the NBDs bind and hydrolyse ATP and that ATP hydrolysis is coupled to transport [18–21]. Elegant studies of the mammalian multidrug resistance P-gp by Alan Senior defined the biochemistry of the ATPase cycle and showed that the two catalytic sites are both required for transport function and that they hydrolyse ATP alternately [22]. Subsequently, several related models for transport appeared based principally on data obtained for the bacterial histidine and maltose transporters and the mammalian P-gp. Given that the two ATP-binding pockets hydrolyse ATP non-simultaneously, these models generally assumed that the two NBDs were coupled to distinct steps in the transport cycle, for example, one ATP hydrolysis might translocate substrate and the second might reset the transporter.
Three recent datasets, however, have caused these models to be revisited. First, biochemical data suggesting that ATP-binding, without hydrolysis, can provide sufficient energy for transport. Second, structural data (see above) showing that the two ATP-binding pockets are not independent but are located at the interface of an NBD dimer ‘sandwich’, implying that the two NBDs act in concert at a single step rather than influencing distinct steps in the transport cycle. Third, the observation that the ATP binding and hydrolysis activities of the two catalytic sites of some ABC transporters (e.g. CFTR and MRP1 of the ABCC family) can differ dramatically.
The “ATP-switch” model
The “ATP-switch” model [3] is based on structural and biochemical data from a number of ABC transporters. The critical facet of this model is that transport is a multistep process involving communication via conformational changes, in both directions, between the NBDs and TMDs (Fig. 3). The driver for transport is an on–off ‘switch’ between two principal conformations of the NBDs: a ‘closed dimer’ formed by binding two ATP molecules at the dimer interface, and dissociation of the ‘closed dimer’ to an ‘open dimer’ facilitated by ATP hydrolysis and Pi/ADP release. The ‘switch’ from the ‘open’ to ‘closed’ conformation of the NBD dimer induces conformational changes in the TMDs necessary for transport (or, for some ABC proteins, biological processes other than transport). The reverse of this switch, from the ‘closed’ dimer to ‘open’ dimer after ATP hydrolysis, resets the transporter ready for the next transport cycle. The model described below is for an exporter such as P-gp, specifically export of the drug vinblastine (the flexibility of the TMDs to export different substrates with different kinetics is considered in the following section). Variations in the kinetics and control of the switch enables differential control of rates and directionality of transport seen for different ABC transporters. Finally, because of the differences between the TMDs of different ABC transporters, it is not unreasonable to suppose that, even though the ‘switch’ may be similar for all ABC transporters, the different TMDs of transporters may respond differently to the energy released at different stages of ATP catalysis.
Schematic of the ‘ATP switch’ model for the transport cycle of an ABC transporter. The schematic is for export of vinblastine by P-gp adapted from Higgins and Linton [3]. The TMDs are shown as ‘cylinders’ spanning the membrane; the NBDs as ‘shapes’ at the cytoplasmic face of the membrane. The transporter in its basal state (top left) has the NBDs in ‘open dimer’ configuration with low affinity for ATP and a high-affinity, vinblastine-binding site in the TMDs (yellow star) exposed to the inner leaflet of the membrane. Step I The transport cycle is initiated by binding of substrate to its high-affinity site. This sends a signal to the NBDs, which facilitates co-operative binding of two molecules of ATP and closed NBD dimer formation, either by allowing de novo binding or by increasing the affinity for pre-bound ATP. Step II The conformational changes required to form the ‘closed NBD dimer’ are transmitted to the TMDs, such that the vinblastine-binding site is exposed extracellularly and its affinity for vinblastine is reduced. Vinblastine is released extracellularly. Step III ATP is hydrolysed to form a transition state intermediate. Hydrolysis of the two ATP molecules is normally sequential (although for some ABC transporters, only one ATP may be hydrolysed). Step IV Sequential release of Pi, and then ADP, restores the transporter to its basal configuration. As discussed in the text, ABC transporters may differ in the detail of the cycle. For example, in some cases, one NBD appears to provide the preferred site of ATP binding or hydrolysis, whilst for other ABC transporters, the selection may be stochastic. For some transporters, hydrolysis of only one ATP molecule may be required. The point of substrate release may also vary. Nevertheless, despite such variations, the transition between ‘open’ and ‘closed’ NBD dimer, and associated conformational changes, provides a common mechanism for transport
Step I. The transport cycle is initiated by binding of substrate to the TMDs with the NBDs in the ‘open dimer’ conformation. A conformational change is transmitted to the NBDs facilitating ATP binding and ‘closed dimer’ formation
In this model, an ABC transporter in its basal state has low affinity for ATP, and the NBDs are in the ‘open dimer’ configuration. A high-affinity binding site (or sites; see below) for the transport substrate on the TMDs is accessible from the cytoplasmic face of the membrane. Logically, substrate binding to the transporter must initiate the transport cycle—otherwise, the binding and hydrolysis of ATP will occur independently of transport, and the cycle will be futile. Thus, the transport cycle is initiated by the transport substrate binding to its high-affinity binding site(s) on the TMDs. This induces a conformational change which is transmitted to the NBDs, facilitating ATP binding and transition from the ‘open’ to ‘closed’ NBD dimer.
The most unambiguous evidence that binding of the transport substrate initiates the transport cycle comes from characterisation of mutants of the bacterial histidine and maltose transporters. In these mutants, the requirement for binding substrate (histidine or maltose complexed with their respective PBPs) to initiate transport is lost, and, consequently, ATP is hydrolysed continuously in a futile cycle [23, 24]. It is of interest that whilst some of these mutants are in the NBDs, others are in the TMDs demonstrating that signals from the TMDs are transmitted to the NBDs to initiate the ATP catalysis cycle. For many other ABC transporters, it has also been demonstrated that substrate binding to the TMDs induces a conformational change in the NBDs. For example, spectroscopic and protease/chemical accessibility studies have shown substrate-induced conformational changes in the NBDs of P-gp [25, 26], TAP (ABCB2/3) [27], MRP1 (ABCC1) [28] and the bacterial histidine [29] and maltose [30] permeases. Substrate binding to the TMDs has also been shown to stimulate ATP hydrolysis by the NBDs, first for the bacterial histidine and oligopeptide permeases [20, 21] and mammalian P-gp [31], and subsequently for many other ABC transporters. Similarly, for the DNA repair ABC protein MutS, substrate (‘mismatch’ DNA) stimulates ATPase activity of the protein [32]; and for bacterial PBP-dependent transporters, interaction of the PBP–substrate complex with the TMDs initiates the transport cycle in an analogous fashion to the interaction of substrate with exporters [33].
It has been difficult to distinguish experimentally whether substrate-induced ATPase activity is due to stimulation of ATP binding or of the hydrolytic step itself. The available data suggest that it is binding of ATP to the NBDs which is enhanced, lowering the activation energy for ‘closed NBD dimer’ formation. First, a mutant E. coli maltose transporter in which the requirement for substrate to stimulate ATP hydrolysis has been lost [24] has a 30-fold increase in NBD affinity for ATP [30]. Secondly, for mammalian P-gp, the transport substrate vinblastine increases the apparent affinity of the NBDs for ATP nearly 60-fold (Linton, Wooding and Higgins, submitted for publication), and fluorescent probes for nucleotide binding have shown that several drugs increase the affinity for ATP [34]. Although, in other studies of P-gp, drug substrates have been reported not to alter the affinity for ATP [22, 31], in these studies, the affinity for ATP was indirectly inferred from increased ATPase activity above a basal activity in the absence of added substrate; this basal activity itself reflects the anticipated futile cycle (see above), and so the data cannot be interpreted as intended. Finally, binding of substrate (‘mismatch’ DNA) to the DNA-binding domain (analogous to the TMDs of an ABC transporter) of the MutS mismatch repair enzyme stimulates the NBD to bind ATP [35, 36].
The nature of the conformational changes involved in transmitting a signal from the TMDs to the NBDs to enhance ATP binding and ‘closed dimer’ formation remains speculative, although it seems likely that the L-loop (EAA sequence) of TMDs [6, 37] and the Q-loops [10] and ‘structurally diverse’ regions [38] of the NBDs are involved. Similarly, we have little idea of the conformational changes which facilitate ATP binding by the NBDs. It has been suggested from structures of both the HlyB-NBD and the GlcV NBD in their nucleotide-free forms [39, 40] that unusual conformations of the Walker A loop occlude the nucleotide-binding pockets, and displacement of this loop in response to a signal transmitted from the TMDs might increase affinity for ATP. Alternatively, and perhaps more probably, ATP may always have access to, and ability to bind with low affinity to, the NBDs in the ‘open dimer’ configuration, but a conformational change transmitted from the TMDs is required to align the ‘ABC signature motifs’ from the opposing NBD to form a nucleotide-binding pocket of high affinity and enable ‘closed NBD dimer’ formation. ABC transporters may differ in this respect depending upon the relative basal affinity for ATP in the absence of transport substrate.
Step II. Formation of the ‘closed NBD dimer’ around the bound ATP molecules induces a major conformational change in the TMDs to initiate substrate (vinblastine) translocation
Export across a cell membrane requires that a high-affinity binding site for the transport substrate accessible from the cytoplasmic face of the membrane is converted into a low-affinity site at the extracellular face of the membrane. For an active transporter, these conformational changes require energy input, and, until recently, it was generally assumed that the free energy of ATP hydrolysis drove these conformational changes [41]. However, it is now becoming clear that ATP binding and ‘closed dimer’ formation, rather than hydrolysis, provide sufficient energy to induce the key conformational changes involved in substrate transport.
The observations that amino acids from both NBDs coordinate with each ATP and that around half the area buried at the dimer interface is contributed by the two ATP molecules allow few alternatives to the hypothesis that ATP binding drives ‘closed dimer’ formation and contributes substantially to its stability [9, 10, 42]. The precise conformational changes which trigger ‘closed dimer’ formation remain unclear because structures with and without bound nucleotide have only been obtained for NBDs in the absence of TMDs. Biochemical studies of intact transport complexes suggest that the conformational changes at the NBDs are relatively small [43, 44], implying a transition between an ‘open NBD dimer’ and ‘closed NBD dimer’ configuration rather than a major reorientation of the NBDs with respect to other domains. Free NBDs in solution appear relatively flexible in the absence of ATP [39, 42] and cannot form a stable dimer as the buried interface would be relatively small. By comparing the structures of nucleotide-free monomers and ATP-bound dimers (only possible with mutant NBDs with key changes to prevent hydrolysis), it has become clear that high-affinity ATP binding involves ‘rigid body’ rotation of the α-helical subdomain with respect to the core subdomain (9, 10, 42; Fig. 1c). This aligns residues in the ‘ABC signature’ motif of the α-helical subdomain of one NBD with the Walker A and B motifs of the core subdomain of the other NBD to form two complete ATP-binding pockets. There is good evidence for several ABC transporters that binding of the two ATP molecules is co-operative, providing kinetic control of transport.
It is now also clear that the substantial free energy associated with ATP binding [10, 40] and ‘closed dimer’ formation induces major conformational changes in the TMDs. Spectroscopic, protease accessibility and cross-linking studies have shown that ATP binding to the NBDs induces conformational changes in the TMDs of many ABC transporters. These conformational changes have been visualised directly for P-gp by 2-D crystallography [5, 14]. Comparison of 2-D crystal structures of ATP (AMP-PNP)-bound P-gp with those of P-gp trapped in the post-hydrolytic transition state (with vanadate; see below) showed that the major conformational change in the TMDs occurs upon ATP binding and that subsequent ATP hydrolysis and ADP/Pi release introduce more limited changes [5]. Spectroscopic studies of LmrA lead to a similar conclusion [45]. The nature of these conformational changes remains to be elucidated, although there is clearly a substantial repacking of the TMDs throughout the depth of the membrane [5, 14]. Experimental evidence suggests that threading of α-helices into and out of the lipid bilayer is unlikely [46]. Rotation and tilting of transmembrane α-helices may both contribute to these conformational changes, although, given the diversity of TMDs, it is likely that different types of conformational change are involved for different sub-families of ABC transporters.
Finally, and most importantly, ATP binding can induce changes in the substrate-binding properties of the TMDs. The affinity of substrates for ABC transporters has been difficult to measure directly, and indirect measurements (i.e. stimulation of ATPase activity) often reflect other rate-limiting steps. However, direct measurement of the binding affinity of the drug vinblastine to P-gp has been possible. The use of non-hydrolysable ATP analogues (both AMP-PNP and ATP-γ-S) showed that ATP binding, in the absence of hydrolysis, is sufficient to reduce substrate-binding affinity [5, 47, 48] with little effect on binding capacity or affinity for a modulator which binds to an allosteric site but is not transported, arguing against non-specific disruption of protein structure. For the cystic fibrosis transmembrane regulator, although initial studies suggested that ATP hydrolysis drives channel opening, a more detailed analysis has revealed that channel opening can be mediated by ATP binding to the NBDs in the absence of hydrolysis [49]. Similarly, for the DNA ‘mismatch’ repair ABC proteins, MutS and hMSH2/6, ATP binding in the absence of hydrolysis induces the key conformational change which enables the DNA repair complex to assemble [32, 35, 36].
Step III. ATP is hydrolysed to initiate transition of the NBD ‘closed dimer’ to the ‘open dimer’ configuration and return the transporter to its basal state
ABC transporters normally hydrolyse ATP as part of the transport cycle. ATP hydrolysis destabilises the NBD ‘closed dimer’ to initiate, resetting the transporter to its basal state. The trigger which initiates ATP hydrolysis is unknown. Substrate release from the TMDs could trigger a conformational change which is transmitted to the NBDs, but this is unlikely to be a common mechanism (see below “When does substrate cross the membrane...?”). More likely, ATP hydrolysis is an automatic consequence of ‘closed NBD dimer’ formation. The observation that stable ‘closed dimers’ of isolated NBDs are difficult to obtain in the presence of ATP [50, 39] suggests that the NBDs may be autocatalytic, and crystals of the isolated LolD and HlyB-NBD homodimers with bound ATP could only be obtained once specific mutations preventing hydrolysis were introduced. The structure of the mutant LolD homodimer in its ATP-bound state shows a water molecule positioned with ideal geometry for hydrolytic attack on the γ-phosphate group of ATP, but which cannot be activated because the required H-bond acceptor, the catalytic base E171, has been mutated stabilising the structure ([10]; Fig. 4a). However, structures of similar mutants of HlyB-NBD with bound ATP have led to a different interpretation of the molecular mechanism of hydrolysis. Arguing that base catalysis would be possible in monomeric NBDs and that free NBDs in solution need to dimerise to hydrolyse ATP, Schmitt et al. [51] have proposed a substrate-assisted catalysis (Fig. 4b). This requires the microenvironment of the closed NBD dimer to enable ATP itself to abstract a hydrogen from the catalytic water and activate it for nucleophilic attack on the γ-phosphate. Presently, it is not possible to be unequivocal about the mechanism of catalysis, particularly given that the current dataset cannot assess the possible role for the TMDs in the process [10].
Possible mechanisms of ATP hydrolysis. a General base catalysis. The histidine of the H-loop donates a hydrogen bond to the γ-phosphate of ATP stabilizing its position. The invariant glutamate of the Walker B motif is oriented towards the water from which it abstracts a proton (arrow), thus activating the water for nucleophilic attack on the γ-phosphate. b Substrate-assisted catalysis. The H-loop histidine donates the same hydrogen bond to the γ-phosphate of ATP, but the role of the Walker B glutamate is to accept two hydrogen bonds from the H-loop histidine stabilizing the position of the latter in the active site. Catalysis is “substrate-assisted” as the γ-phosphate acts as a base to abstract a proton from the hydrolytic water (arrow), activating it for nucleophilic attack. Such a substrate-assisted mechanism would be dependent on the pKa of the ATP which in turn would be dependent on the local environment of the active site. Images were generated in MacPyMOL using the pdb (1XEF) of the H662A mutant of HlyB as a template [51]. Only one NBD is shown for clarity; the side chains of Glu631 and His662, the proposed hydrolytic water and the ATP are shown in stick form and coloured elementally. The magnesium cofactor is shown as a green sphere
After hydrolysis, the released Pi exits the NBD dimer and can be replaced by vanadate, a potent inhibitor of ABC transporter function. Vanadate is able to stabilise the NBD dimer with one ATP and one ADP.Vi in a transition state. Biochemical evidence suggests that this is an activated state [52] with a distinct conformation of the TMDs of P-gp, which retains a low affinity for vinblastine [5, 53]. Comparison of the high-resolution structures of ATP- and ADP-bound HlyB-NBD [40] suggests that some of the energy of hydrolysis be used to distort a helix (number 6; Fig. 1c) in the NBD storing energy to force the later dissociation of ADP (see below). Hydrolysis of ATP, therefore, does not restore the transporter to its basal state but is a necessary step in the restoration of the basal state.
Step IV. Pi and then ADP are released, restoring the protein to its basal state ready to initiate another transport cycle
After ATP hydrolysis, Pi must be released before ADP because Vi is able to replace Pi and stabilise the NBD dimer in complex with ADP [22, 54, 55]. How Pi is released from the post-hydrolytic complex is again contentious. Based on comparison of the electrostatic potential of monomeric and dimeric LolD, it has been proposed that electrostatic repulsion between the ADP coordinated by the core subdomain of one NBD and the Pi coordinated by the signature motif of the other NBD destabilises the closed NBD dimer, leading to Pi and ADP release [10]. A different mechanism has been postulated for the isolated HlyB-NBD. Hydrophilic tunnels have been described for the mutant HlyB-NBDs with bound ATP [40], and it is suggested that these represent exit tunnels for the released Pi. The tunnels are asymmetric in the HlyB-NBD homodimeric structure. One tunnel provides a continuous passage from one γ-phosphate to the surface of the dimer; the other tunnel, extending from the γ-phosphate of the second ATP, is closed by a salt bridge providing an explanation for the observed, non-simultaneous hydrolysis of the two ATPs in the complex. Thus, it is postulated that Pi leaves the closed HlyB-NBD dimer via an exit tunnel without a build up of electrostatic charge. The remaining ADP is unable to stabilise the NBD dimer, and the dissociation of the NBDs results after which the energy stored in the torsion of helix 6 is released to displace ADP. This mechanism may not be common to all ABC NBDs, and, indeed, for MutS, ADP appears to remain bound to one NBD until substrate binding induces a conformational change in the NBDs, such that ADP is displaced by ATP [36]. For P-gp [22, 56, 57] and MutS [35], it has been shown that ADP release provides a rate-limiting step in the ATPase cycle.
When does transport substrate cross the membrane and when is it released extracellularly during the transport cycle?
Substrate translocation across the membrane requires reorientation of the substrate-binding site. This has been hard to measure directly. Only for the bacterial drug transporter LmrA have direct measurements of orientation been made [58]. The high-affinity site is accessible from the inner leaflet of the membrane in the native state, as expected. Furthermore, the low-affinity site is exposed to the extracellular medium in the post-hydrolytic state (Vi-trapped) showing that reorientation and drug release must have occurred before ‘closed dimer’ dissociation. However, this does not distinguish at which earlier step reorientation of the binding site occurs.
The ‘power stroke’ for transport is a functional definition and does not necessarily imply that this is the sole step for energy input into the system. Clearly, as ATP binding, ATP hydrolysis and Pi/ADP release each cause significant conformational changes in the TMDs [5], and each therefore inputs energy into the transport cycle. At least for P-gp, it is becoming apparent that this may have different consequences for different drug-binding sites (Fig. 5) and may resolve apparent discrepancies in the literature. P-gp has multiple drug-binding sites which may behave differently (see below). In the absence of nucleotide, P-gp has a measurable high affinity for three different drugs: vinblastine, as described above [48], IAAP [57] and propafenone (Chiba, personal communication). The conformational changes in the TMDs after binding of a non-hydrolysable analogue of ATP and ‘closed NBD dimer’ formation result in reduced affinity of the transporter for vinblastine and propafenone, but the affinity for IAAP is reported to remain high. Furthermore, the affinity for IAAP is only lowered during the conformational change in response to ATP hydrolysis as measured in the ADP/Vi-trapped state. The high affinity for propafenone is also restored at this step, but high affinity for vinblastine is not recovered until phosphate is released from the NBDs. Thus, at least for P-gp, the three energetic steps in ATP catalysis (ATP binding, ATP hydrolysis and Pi/ADP release) are all associated with conformational changes in the transporter, and each may be used to reconfigure the binding sites for different drugs at different steps of the ATPase catalytic cycle.
The ‘ATP switch’ induces multiple conformational changes in the TMDs of ABC transporters, all of which contribute to transport substrate across the membrane. The TMDs change conformation in response to the energy released at different steps of the ATP catalytic cycle at the NBDs. This may have different consequences for different substrate-binding sites. In the multispecific drug–transporter, P-gp, the binding sites for vinblastine (Vbl), IAAP and propafenone (Prop) are all in a high affinity state (red) in the absence of bound nucleotide, allowing drugs to bind from an intracellular aspect. The affinity for both vinblastine and propafenone is reduced (blue) after ATP binding to the TMDs, and these drugs are, therefore, presumably released on the extracellular side of the membrane at this step. However, the affinity for IAAP remains unchanged upon ATP binding, and high affinity is not lost until ATP is hydrolysed. High affinity for propafenone is also restored at this step but, in contrast, high affinity for vinblastine is not recovered until phosphate is released from the NBDs. It therefore seems that the conformational changes in the TMDs, at different steps in the catalytic cycle, may have different consequences for the different drug-binding sites
Stoichiometry of ATP binding and hydrolysis
All ABC transporters have two putative nucleotide-binding pockets. Two ATP molecules are normally bound at the dimer interface in the ‘closed dimer’—two bound nucleotides have been observed in crystallographic dimers of mutant LolD [10] and HlyB-NBD [51], MalK [42] and Rad50 [9]; have been detected biochemically in the Mdl1p dimer [55]; and have been shown, biochemically, to be bound by the intact MRP1 and P-gp transporters [34, 52]. For CFTR, there is indirect evidence that ATP must bind to both sites to open the channel [59, 60]. Finally, co-operative ATP binding suggests a role for two ATP molecules [50, 61–64]. However, it is possible to envisage an ABC transporter where one ATP is sufficient to stabilise the ‘closed dimer’ depending on the nature of the dimer interface. The ATP switch model does not require one or other nucleotide-binding pocket to bind ATP first. For some transporters, the decision may be stochastic (i.e. for P-gp; [63, 65]). However, for both MRP1 [66] and SUR1 (ABCC8; [67]), there is evidence that NBD1 provides the initial ATP-binding site. These differences may provide different kinetic control to ‘closed dimer’ formation and, hence, transport.
Whether both ATP molecules are hydrolysed is less clear. For many ABC transporters, both nucleotide-binding pockets have the capacity to hydrolyse ATP and clearly demonstrated for P-gp [68, 69], TAP [70] and the histidine permease [71]. For some ABC transporters, the two pockets hydrolyse ATP non-simultaneously, for example, P-gp [22, 52, 63, 72], MutS [36], and the bacterial histidine [50, 61] and maltose [73] transporters. Consistent with this, the two nucleotide-binding pockets of a transporter frequently exhibit structural asymmetry even for HisP [29] and HlyB-NBD [40] where the two NBDs are identical in sequence. Alternating hydrolysis has previously been interpreted as implying that the two hydrolysis events affect different steps of the transport cycle (i.e. there is no redundancy). It now seems more likely that co-operative ATP hydrolysis enhances the kinetics of ‘closed dimer’ dissociation. Support for this interpretation comes from the DNA repair ABC protein MutS. Replacement of a single amino acid R697A at the NBD dimer interface, which interacts with the opposing NBD, abolishes alternating hydrolysis and reduces the efficiency of DNA repair [36]. For some ABC transporters, the ‘choice’ of which NBD hydrolyses first may be stochastic [65]. For others, there is a clear asymmetry: for MRP1, NBD2 appears to hydrolyse ATP initially [66, 74], and in CFTR, hydrolysis of one ATP is much slower than the other [84]. Nevertheless, the net result is the same: destabilisation of the ‘closed dimer’ albeit with different kinetic control. It is also not impossible to envisage that ATP hydrolysis at only one pocket might be sufficient to destabilise the ‘closed dimer’, albeit at a slower rate. Indeed, it has been shown by experimental manipulation that ATP hydrolysis by both pockets is not absolutely essential for transport. Thus, for the histidine transporter, mutation of one pocket to abolish ATP hydrolysis, without inhibiting ATP hydrolysis by the other pocket, still enables histidine transport albeit at a reduced rate [71]. For CFTR, although ATP is normally hydrolysed with faster kinetics by one pocket over the other, hydrolysis is not absolutely required by either NBD but simply appears to enhance the rate of nucleotide release and formation of the ‘open dimer’ [59]. Although this might be peculiar to an ion channel, where rates of channel opening and closing do not need to be rapid, some ABC transporters lack key residues in the ‘ABC signature’ motif of one NBD which also suggests that hydrolysis of only one ATP may be required.
In conclusion, therefore, it appears that two ATP molecules are commonly bound and hydrolysed during a transport cycle in a co-operative fashion. However, for some ABC transporters, only a single hydrolytic event may destabilise the ‘closed NBD dimer’ and restore the transporter to its basal state.
Substrate-binding sites
The substrate specificity of ABC transporters is generally determined by one or more substrate-binding sites located on the TMDs. In the absence of structural data with bound substrate, little is known about the nature of these sites or their precise locations on the TMDs. Numerous mutagenesis and cross-linking studies have implicated large regions of the TMDs; however, with mutagenesis, it is difficult to rule out allosteric effects, and with cross-linking studies, size and promiscuity of the cross-linker may be a problem. Some ABC transporters only transport one molecule of substrate per transport cycle, presumably involving a single site. For example, TAP appears to have a single peptide-binding site with the N terminus of the translocated peptide binding to one TMD and the C terminus to the other TMD [75]. In contrast, other ABC transporters have multiple substrate-binding sites, particularly drug transporters with broad specificity. For example, some MRPs can transport both cytotoxic drugs and GSH, and these bind to distinct binding sites [76, 77]. As they can also transport drug--GSH conjugates, the number of sites may be a semantic point; one site for a drug–GSH conjugate may become two sites when the ligands are not conjugated. Similarly for P-gp, detailed pharmacological studies have identified three interacting substrate-binding sites from which transport can occur [78, 79]. Indeed, it has been shown [80] that two drug molecules can bind to a single protein molecule at the same time. Frequently, one substrate can stimulate transport of the other [58, 81, 82], possibly because occupancy of two sites more effectively stimulates ATP binding and ‘closed NBD dimer’ formation. In support of this hypothesis, GSH and drug must both bind to MRP1 to efficiently induce activating conformational changes in the NBDs [28]. It seems likely that for some ABC transporters, two ATP molecules are hydrolysed per transport cycle whilst for others, it is only one (see above). Similarly, as some transporters have multiple sites, all of which may not always all be occupied, the stoichiometry may vary depending on the substrate(s) studied. In some cases, for example haemolysin A, a large (40 kDa) polypeptide transported across the inner membrane, periplasmic space and outer membrane of E. coli by the HlyB, HlyD and TolC complex may require rather more than two ATPs to be hydrolysed in repetitive rounds of conformational change.
Summary
Biochemical, structural and genetic data from many laboratories, and based on several different ABC transporters, have led to the ‘ATP-switch’ model. The model describes the basic principles by which an ABC transporter operates and can accommodate apparent differences between ABC transporters. These differences lie in two major areas. First, whether the two NBDs are equivalent in their ability to bind and hydrolyse ATP. The hypothesis that the transition between an ‘open’ and ‘closed’ NBD dimer is the key ‘switch’ in the transport process allows for adaptations in which binding/hydrolysis of either one or both ATP molecules may induce ‘closed’/’open’ dimer formation, permitting differential kinetic control of the transport process. Second, the model allows the same ATP-dependent ‘switch’ to have different consequences depending on the TMDs to which it is coupled. Thus, some ABC transporters can import whilst others export. For some, multiple substrates can be transported but the precise step at which substrate is released in the transport cycle may vary. It is also apparent that the TMDs of different ABC transporters have different evolutionary origins. We also now know of several ABC proteins which exploit the same ‘ATP switch’ to control biological processes other than transport making the ABC dimer a controllable switch, adaptable to many requirements.
References
Holland IB, Cole SPC, Kuchler K, Higgins CF (2003) ABC proteins: from bacteria to man. Academic Press, London
Higgins CF, Haag PD, Nikaido K, Ardeshir F, Garcia G, Ames GF-L (1982) Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature 298:723–727
Higgins CF, Linton KJ (2004) The ATP switch model for ABC transporters. Nat Struct Mol Biol 11:918–926
Higgins CF, Hiles ID, Salmond GPC, Gill DR, Downie JA, Evans IJ, Holland IB, Gray L, Buckel SD, Bell AW, Hermodson MA (1986) A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323:448–450
Rosenberg MF, Velarde G, Ford RC, Martin C, Berridge G, Kerr ID, Callaghan R, Schmidlin A, Wooding C, Linton KJ, Higgins CF (2001) Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J 20:5615–5625
Locher KP, Lee AT, Rees DC (2002) The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296:1091–1098
Chang G, Roth CB (2001) Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293:1793–1800
Hung LW, Wang IX, Nikaido K, Liu PQ, Ames GF, Kim SH (1998) Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 396:703–707
Hopfner K-P, Karcher A, Shin DS, Craig L, Arthur M, Carney JP, Tainer JA (2000) Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789–800
Smith PC, Karpowich N, Millen L, Moody JE, Rosen J, Thomas PJ, Hunt JF (2002) ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell 10:139–149
Lamers MH, Perrakis A, Enzlin JH, Winterwerp HHK, de Wind N, Sixma TK (2000) ATP increases the affinity between MutS ATPase domains. Implications for ATP hydrolysis and conformational changes. Nature 407:711–717
Obmolova G, Ban C, Hsieh P, Yang W (2000) Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature 407:703–710
Rosenberg MF, Callaghan R, Ford RC, Higgins CF (1997) Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem 272:10685–10694
Rosenberg MF, Kamis AB, Callaghan R, Higgins CF, Ford RC (2003) Three-dimensional structures of the mammalian multidrug resistance P-glycoprotein demonstrate major conformational changes in the transmembrane domains upon nucleotide binding. J Biol Chem 278:8294–8299
Chang G (2003) Structure of MsbA from Vibrio cholera: a multidrug resistance ABC transporter homolog in a closed conformation. J Mol Biol 330:419–430
Reyes CL, Chang G (2005) Structure of the ABC transporter MsbA in complex with ADP vanadate and lipopolysaccharide. Science 308:1028–1031
Stenham DR, Campbell JD, Sansom MSP, Higgins CF, Kerr ID, Linton KJ (2003) An atomic detail model for the human ATP binding cassette transporter P-glycoprotein derived from disulfide cross-linking and homology modeling. FASEB J 15:2287–2289
Hobson AC, Weatherwax R, Ames GF-L (1984) ATP-binding sites in the membrane components of histidine permease, a periplasmic transport system. Proc Natl Acad Sci USA 81:7333–7337
Higgins CF, Hiles ID, Whalley K, Jamieson DJ (1985) Nucleotide binding by membrane components of bacterial periplasmic binding protein-dependent transport systems. EMBO J 4:1033–1040
Bishop L, Agbayani R, Ambudkar SV, Maloney PC, Ames GF-L (1989) Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc Natl Acad Sci USA 86:6953–6957
Mimmack ML, Gallagher MP, Hyde SC, Pearce SR, Booth IR, Higgins CF (1989) Energy coupling to periplasmic binding protein-dependent transport systems: stoichiometry of ATP hydrolysis during transport in vivo. Proc Natl Acad Sci USA 86:8257–8261
Senior AE, Al-Shawi MK, Urbatsch IL (1995) The catalytic cycle of P-glycoprotein. FEBS Lett 377:285–289
Petronilli V, Ames GF-L (1991) Binding protein-independent histidine permease mutants. Uncoupling of ATP hydrolysis from transmembrane signaling. J Biol Chem 266:16293–16296
Davidson AL, Shuman HA, Nikaido H (1992) Mechanism of maltose transport in Escherichia coli: transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci USA 89:2360–2364
Liu R, Sharom FJ (1996) Site-directed fluorescence labeling of P-glycoprotein on cysteine residues in the nucleotide binding domains. Biochemistry 35:11865–11873
Sonveaux N, Vigano C, Shapiro AB, Ling V, Ruysschaert J-M (1999) Secondary and tertiary structure changes of reconstituted P-glycoprotein. A Fourier transform attenuated total reflection infrared spectroscopy analysis. J Biol Chem 274:17649–17654
Neumann L, Abele R, Tampé R (2002) Thermodynamics of peptide binding to the transporter associated with antigen processing (TAP). J Mol Biol 324:965–973
Manciu L, Chang X-B, Hou Y-X, Gustot A, Riordan JR, Ruysschaert JM (2003) Intermediate structural states involved in MRP1-mediated drug transport. Role of glutathione. J Biol Chem 278:3347–3356
Kreimer DI, Chai KP, Ames GF-L (2000) Nonequivalence of the nucleotide-binding subunits of an ABC transporter, the histidine permease, and conformational changes in the membrane complex. Biochemistry 39:14183–14195
Mannering DE, Sharma S, Davidson AL (2001) Demonstration of conformational changes associated with activation of the maltose transport complex. J Biol Chem 376:12362–12368
Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA (1992) Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J Biol Chem 267:4854–4858
Gradia S, Acharya S, Fishel R (1997) The human mismatch recognition complex hMSH2–hMSH6 functions as a novel molecular switch. Cell 91:995–1005
Austermuhle MI, Hall JA, Klug CS, Davidson AL (2004) Maltose-binding protein is open in the catalytic transition state for ATP hydrolysis during maltose transport. J Biol Chem 279:28243–28250
Qu Q, Russell PL, Sharom FJ (2003) Stoichiometry and affinity of nucleotide binding to P-glycoprotein during the catalytic cycle. Biochemistry 42:1170–1177
Gradia S, Subramanian D, Wilson T, Acharya S, Makhov A, Griffith J, Fishel R (1999) hMSH2–hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol Cell 3:255–261
Lamers MH, Winterwerp HHK, Sixma TK (2003) The alternating ATPase domains of MutS control DNA mismatch repair. EMBO J 22:746–756
Mourez M, Hofnung M, Dassa E (1997) Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J 16:3066–3077
Schmitt L, Benabdelhak H, Blight MA, Holland IB, Stubbs MA (2003) Crystal structure of the nucleotide-binding domain of the ABC-transporter haemolysin B: identification of a variable region within ABC helical domains. J Mol Biol 330:333–342
Verdon G, Albers SV, Dijkstra BW, Driessen AJM, Thunnissen A-M (2003) Crystal structures of the ATPase subunit of the glucose ABC transporter from Sulfolobus solfataricus: nucleotide-free and nucleotide-bound conformations. J Mol Biol 330:343–358
Zaitseva J, Oswald C, Jumpertz T, Jenewein S, Wiedenmann A, Holland IB, Schmitt L (2006) A structural analysis of asymmetry required for catalytic activity an ABC ATPase domain dimer. EMBO J 25:3432–3443
Mitchell P (1957) A general theory of membrane transport from studies of bacteria. Nature 180:134–136
Chen J, Lu G, Lin J, Davidson AL, Quiocho FA (2003) A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol Cell 12:651–661
Liu R, Sharom FJ (1997) Fluorescence studies on the nucleotide binding domains of the P-glycoprotein multidrug transporter. Biochemistry 36:2836–2843
Loo TW, Clarke DM (2000) Drug-stimulated ATPase activity of human P-glycoprotein is blocked by disulfide cross-linking between the nucleotide-binding sites. J Biol Chem 275:19435–19438
Vigano C, Margolles A, van Veen HW, Konings WN, Ruysschaert J-M (2000) Secondary and tertiary structure changes of reconstituted LmrA induced by nucleotide binding or hydrolysis. A fourier transform attenuated total reflection infrared spectroscopy and tryptophan fluorescence quenching analysis. J Biol Chem 275:10962–10967
Linton KJ, Higgins CF (2002) P-glycoprotein misfolds in Escherichia coli: evidence against alternating-topology models of the transport cycle. Mol Membr Biol 19:51–58
Martin C, Berridge G, Higgins CF, Mistry P, Charlton P, Callaghan R (2000) Communication between multiple drug binding sites on P-glycoprotein. Mol Pharmacol 58:624–632
Martin C, Higgins CF, Callaghan R (2001) The vinblastine binding site adopts high- and low-affinity conformations during a transport cycle of P-glycoprotein. Biochemistry 40:15733–15742
Vergani P, Lockless SW, Nairn AC, Gadsby DC (2005) CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature 433:876–880
Nikaido K, Liu P-Q, Ames GF-L (1997) Purification and characterization of HisP, the ATP-binding subunit of a traffic ATPase (ABC transporter), the histidine permease of Salmonella typhimurium. Solubility, dimerization, and ATPase activity. J Biol Chem 272:27745–27752
Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L (2005) H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J 24:1901–1910
Urbatsch IL, Tyndall GA, Tombline G, Senior AE (2003) P-glycoprotein catalytic mechanism: studies of the ADP-vanadate inhibited state. J Biol Chem 278:23171–23179
Ramachandra M, Ambudkar SV, Chen D, Hrycyna CA, Dey S, Gottesman MM, Pastan I (1989) Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry 37:5010–5019
Sharma S, Davidson AL (2000) Vanadate-induced trapping of nucleotides by purified maltose transport complex requires ATP hydrolysis. J Bacteriol 182:6570–6576
Janas E, Hofacker M, Chen M, Gompf S, van der Does C, Tampé R (2003) The ATP hydrolysis cycle of the nucleotide-binding domain of the mitochondrial ATP-binding cassette transporter Mdl1p. J Biol Chem 278:26862–26869
Kerr KM, Sauna ZE, Ambudkar SV (2001) Correlation between steady-state ATP hydrolysis and vanadate-induced ADP trapping in human P-glycoprotein. Evidence for ADP release as the rate-limiting step in the catalytic cycle and its modulation by substrates. J Biol Chem 276:8657–8664
Sauna ZE, Ambudkar SV (2000) Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci USA 97:2515–2520
van Veen HW, Margolles A, Müller M, Higgins CF, Konings WN (2000) The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J 19:2503–2514
Aleksandrov AA, Chang X-B, Aleksandrov L, Riordan J (2000) The non-hydrolytic pathway of cystic fibrosis transmembrane conductance regulator ion channel gating. J Physiol 528:259–265
Vergani P, Nairn AC, Gadsby DC (2003) On the mechanism of MgATP-dependent gating of CFTR Cl- channels. J Gen Physiol 120:17–36
Liu CE, Liu P-Q, Ames GF-L (1997) Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter). J Biol Chem 272:21883–21891
Davidson AL, Sharma S (1997) Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J Bacteriol 179:5458–5464
Hrycyna CA, Ramachandra M, Ambudkar SV, Young HK, Pedersen PL, Pastan I, Gottesman MM (1998) Mechanism of action of human P-glycoprotein ATPase activity. Photochemical cleavage during a catalytic transition state using orthovanadate reveals cross-talk between the two ATP sites. J Biol Chem 273:16631–16634
Tombline G, Bartholomew L, Gimi K, Tyndall GA, Senior AE (2004) Synergy between conserved ABC signature Ser residues in P-glycoprotein catalysis. J Biol Chem 279:5363–5373
Hrycyna CA, Ramachandra M, Germann UA, Cheng PW, Pastan I, Gottesman MM (1999) Both ATP sites of human P-glycoprotein are essential but not symmetric. Biochemistry 38:13887–13899
Yang R, Cui L, Hou Y-X, Riordan JR, Chang X-B (2003) ATP binding to the first nucleotide binding domain of multidrug resistance-associated protein plays a regulatory role at low nucleotide concentration, whereas ATP hydrolysis at the second plays a dominant role in ATP-dependent leukotriene C4 transport. J Biol Chem 33:30764–30771
Ueda K, Komine J, Matsuo M, Seino S, Amachi T (1999) Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc Natl Acad Sci USA 96:1268–1272
Urbatsch IL, Beaudet L, Carrier I, Gros P (1998) Mutations in either nucleotide-binding site of P-glycoprotein (Mdr3) prevent vanadate trapping of nucleotide at both sites. Biochemistry 37:4592–4602
Szabó K, Welker E, Bakos E, Müller M, Roninson I, Váradi A, Sarkadi B (1998) Drug-stimulated nucleotide trapping in the human multidrug transporter MDR1. Cooperation of the nucleotide binding domains. J Biol Chem 273:10132–10138
Chen M, Abele R, Tampé R (2003) Peptides induce ATP hydrolysis at both subunits of the transporter associated with antigen processing. J Biol Chem 278:29686–29692
Nikaido K, Ames GF-L (1999) One intact ATP-binding subunit is sufficient to support ATP hydrolysis and translocation in an ABC transporter, the histidine permease. J Biol Chem 274:26727–26735
Urbatsch IL, Sankaran B, Weber J, Senior AE (1995) P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J Biol Chem 270:19383–19390
Davidson AL, Laghaeian SS, Mannering DE (1996) The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J Biol Chem 271:4858–4863
Gao M, Cui HR, Loe DW, Grant CE, Almquist KC, Cole SPC, Deeley RG (2000) Comparison of the functional characteristics of the nucleotide binding domains of multidrug resistance protein 1. J Biol Chem 275:13098–13108
Uebel S, Meyer TH, Kraas W, Kienle S, Jung G, Wiesmüller KH, Tampé R (1995) Requirements for peptide binding to the human transporter associated with antigen processing revealed by peptide scans and complex peptide libraries. J Biol Chem 270:18512–18516
Loe DW, Deeley RG, Cole SPC (1998) Characterization of vincristine transport by the M(r) 190,000 multidrug resistance protein (MRP): evidence for cotransport with reduced glutathione. Cancer Res 58:5130–5136
Rius M, Nies AJ, Hummel-Eisenbeiss J, Jedlitschky G, Keppler D (2003) Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology 38:374–384
Martin C, Berridge G, Higgins CF, Callaghan R (1997) The multi-drug resistance reversal agent SR33557 and modulation of vinca alkaloid binding to P-glycoprotein by an allosteric interaction. Br J Pharmacol 122:765-771
Martin C, Berridge G, Mistry P, Higgins CF, Charlton P, Callaghan R (2000) Drug binding sites on P-glycoprotein are altered by ATP binding prior to nucleotide hydrolysis. Biochemistry 39:11901–11906
Lugo MR, Sharom FJ (2005) Interaction of LDS-751 and rhodamine 123 with P-glycoprotein: evidence for simultaneous binding of both drugs. Biochemistry 44:14020–14029
Zelcer N, Huisman MT, Reid G, Wielinga P, Breedveld P, Kuil A, Knipscheer P, Schellens JH, Schinkel A, Borst PJ (2003) Evidence for two interacting ligand binding sites in human multidrug resistance protein 2 (ATP binding cassette C2). J Biol Chem 278:23538–23544
Shapiro AB, Ling V (1997) Positively cooperative sites for drug transport by P-glycoprotein with distinct drug specificities. Eur J Biochem 250:130–137
Campbell JD, Biggin PC, Baaden M, Sansom MS (2003) Extending the structure of an ABC transporter to atomic resolution: modeling and simulation studies of MsbA. Biochemistry 42:3666–3673
Vergani P, Nairn AC, Gadsby DC (2002) On the mechanism of MgATP-dependent gating of CFTR CI- channels. J Gen Physiol 121:17–36
Acknowledgements
We are grateful for the contributions of many present and former members of the laboratory and collaborators. We also thank many colleagues throughout the world for sharing information and stimulating discussions, particularly Mark Rosenberg, Richard Callaghan, Bob Ford and Rik van Veen, and to Peter Chiba for sharing unpublished data. The authors’ research is funded by the UK Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linton, K.J., Higgins, C.F. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflugers Arch - Eur J Physiol 453, 555–567 (2007). https://doi.org/10.1007/s00424-006-0126-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0126-x