Abstract
Purpose
The aim of this study was to determine if the prognostic value of the preoperative neutrophil-to-lymphocyte ratio (NLR) could be modified by the presence of postoperative complications (POC) and their severity in patients with gastric adenocarcinoma resected with curative intent.
Methods
A retrospective study based on a prospective database of patients with resectable gastric adenocarcinoma treated with radical intention (R0) between January 1998 and February 2012. The primary endpoint was overall survival according to preoperative peripheral blood NLR and postoperative complications. Clinicopathological variables, preoperative blood tests, POC and its severity (Clavien–Dindo classification), type of POC (infectious or not infectious) and mortality were registered. A univariate and multivariate analysis (step forward Cox regression) was performed. The Kaplan–Meier method was used to assess overall survival.
Results
The 147 patients with gastric cancer who had undergone radical resection were included from an initial cohort of 209 patients. Univariant analysis: type of surgery, pT, pN, postoperative complications (Clavien–Dindo ≥ 3) and preoperative NLR ≥ 2.4 were significantly associated with survival (p < 0.05). Patients with POC showed worse long-term survival (p = 0.000), with no difference (p = 0.867) between infectious or non-infectious POC. NLR ≥ 2.4 was associated with infectious POC (p < 0.001). Patients with preoperative NLR ≥ 2.4 (p = 0.02) had a worse prognosis. Multivariate analysis: pN (p < 0.001), postoperative complications (p < 0.001) (HR 3.04; 95% CI: 1.97–4.70) and NLR ≥ 2.4 (p = 0.04) (HR = 1.55; 95% CI: 1.02–2.3) were independent prognostic factors.
Conclusion
The preoperative inflammatory state of patients with gastric cancer measured by NLR behaves as an independent prognostic factor, even in patients with POC.
Similar content being viewed by others
Introduction
It is known that preoperative systemic inflammatory response is related to the development, progression and invasion of tumour cells and consequently to overall survival [1] in most tumours.
Different biological parameters have been identified as markers of a systemic inflammatory state. Some of the most relevant markers are neutrophil-to-lymphocyte ratio (NLR) [2], lymphocyte-to-monocyte ratio (LMR) [3], platelets-to-lymphocytes ratio (PLR) [4], Nutritional Prognosis Index ([10 × albumin] + [0,005 × lymphocytes]) [5], Glasgow Prognosis Score (a combination of C reactive protein and albumin values) [6], systemic immune-inflammatory index (neutrophils × platelets/lymphocytes) [7] and derived neutrophil-to-lymphocyte ratio (neutrophil to white blood cells – neutrophils) [8]. The most frequently analysed biomarker to evaluate systemic inflammatory response is NLR, as it is a simple, easy-to-use and inexpensive tool.
Several studies have shown that a high preoperative neutrophil-to-lymphocyte ratio (NLR) value in peripheral blood tests represents an independent prognostic factor of overall survival in different types of tumours, including gastric cancer [3, 9].
It has also been proven that postoperative complications (especially severe and infectious ones) are independent prognostic factors for long-term survival after curative gastrectomy [10,11,12].
Likewise, preoperative systemic inflammatory response and postoperative complications are both going to affect overall survival, but at different moments of the disease. The preoperative systemic inflammatory state is influenced by the tumour immunity and may induce anti- or pro-tumour response. The presence of postoperative complications (especially more infectious ones) could activate different pro-tumour pathways and impair survival [13].
Given the influence on the survival of preoperative systemic inflammation and systemic inflammation following postoperative complications, both factors should be analysed together to ensure the independent predictive value of each parameter.
The aim of this study was to determine if the prognostic value of the preoperative NLR could be influenced by the presence of postoperative complications in patients with gastric adenocarcinoma resected with curative intent.
Methods
Data collection
This is a retrospective observational study using a prospectively maintained database including all patients treated in the University Hospital Mútua Terrassa (Barcelona, Spain) with gastric adenocarcinoma undergoing gastric resection with curative intent (R0) from January 1998 to February 2012. The study was approved by the ethical committee of our hospital (Acta 10/2019). Exclusion criteria are age under 18 years old, chronic inflammatory or autoimmune illness, neoadjuvant chemotherapy and/or radiotherapy, R1 and R2 resections, intraoperative finding of carcinomatosis and palliative surgery. The preoperative study included gastro-oesophageal endoscopy, thorax-abdomen-pelvis CT and blood tests almost two weeks before surgery. The following preoperative data were systematically collected: age, gender, preoperative ASA grade classification (American Society Anaesthesiologist), neutrophils, lymphocytes, neutrophil-to-lymphocyte ratio (the optimal cut-off value for NLR was obtained according to the median value of the cohort in order to compare higher vs. lower index), tumour location (superior, middle, distal third or gastric linitis), type of gastrectomy (total, subtotal, or other) and type of lymphadenectomy (according to the Japanese Gastric Cancer Guidelines 2017) [14]. All cases were discussed in a multidisciplinary tumour board.
Resected specimens were analysed following the 7th Edition TNM classification [15]. Lauren classification (intestinal, diffuse or mixed) [16] was also specified. Patients with serosa invasion or positive lymph nodes (≥ pT3 and/or ≥ pN1) received adjuvant chemotherapy with mitomycin plus tegafur, according to our hospital protocol.
All postoperative complications (POC) occurring during the first 30 days after surgery were registered, and their severity was graded with the Clavien–Dindo (C-D) classification (grade I–II: minor complications; grade III–IV: major complications which required any interventional treatment or presented organ failure with intensive care support; and grade V: mortality) [17]. Complications were also divided into infectious and non-infectious. Infectious complications were classified according to the previous description published by our group [18]: Sepsis was defined as the presence of two or more systemic inflammatory response syndrome criteria. Intraabdominal infection was defined as (i) an abscess or diffused infection within the abdominal cavity diagnosed by ultrasound or computed tomography, with positive culture; (ii) the presence of an anastomotic leakage (defined as full-thickness gastrointestinal defect involving the anastomosis); (iii) duodenal stump fistula (bile or purulent drainage from a drain placed close to the duodenal stump); (iv) pancreatic fistula (drain output with amylase content) or (v) acute cholecystitis. Respiratory septic complications included pneumonia (defined as new or progressive infiltrate on chest X-ray, accompanied by fever, leukocytosis or leukopenia and purulent sputum, for which antibiotic treatment was started) and pleural empyema.
Wound infection was defined as a deep surgical site infection that required treatment with antibiotics agents or superficial wound drainage. Urinary tract infection was defined according to positive urine culture and clinical findings (fever, urgency, frequency, dysuria or suprapubic tenderness). Finally, catheter-related bloodstream infection was defined as bacteraemia/fungemia in a patient with an intravascular catheter with at least one positive blood culture obtained from a peripheral vein and no other apparent source for the bloodstream infection except the catheter.
Postoperative follow-up was done according to international guidelines [19, 20] with clinical examination 1 month after discharge, a general blood test with tumour markers and clinical examination every six months and annual abdominal CT scan and gastroscopy, until a 5-year follow-up was achieved for initial stages (pTis, pT1N0M0). All other stages were followed with gastroscopy and abdominal CT every six months during the first two years and then annually.
Primary endpoint
The primary endpoint was overall survival, according to preoperative peripheral blood NLR and postoperative complications.
Statistical analysis
Data are shown as frequencies and percentages for categorical variables or as median (interquartile range) for quantitative variables. Chi-square was used to analyse if the patients with higher preoperative NLR had suffered more postoperative complications and more severe complications. Overall survival was calculated from the date of surgery until death from any cause or the date of the last follow-up in living patients. A descriptive statistical analysis and a univariate and multivariable Step Forward Cox regression was performed for the prognostic factors. Kaplan–Meier curves according to NLR with the presence or not of POC were plotted and compared using log-rank statistics. The SPSS software package (SPSS Inc, Chicago, IL, USA), version 25, was used to manage patient data and perform statistical analyses.
Results
Cohort data
Of the 209 patients with resectable gastric cancers, 62 were excluded because they did not meet the inclusion criteria (47 patients) or because they lacked preoperative leukocyte count data (15 patients) (Fig. 1). A total of 147 patients were included for analysis, with a median follow-up of 43.9 months [1–204]. Table 1 shows the clinical and pathological variables of included patients: median age was 67.5 years [33–92], with a majority of men (65%). The most common ASA classifications were ASA-II (68%) and ASA-III (20%); 46% of tumours were distal third; most of the patients were treated with a subtotal gastrectomy and D2 lymphadenectomy (83%). Intestinal adenocarcinoma was the most prevalent Lauren histology type (48.3%), and most of the tumours were locally advanced. POC were registered in 60 patients (41%), 25 (17%) of them were infectious and 32 (22%) were severe (C-D ≥ 3). All POC are listed in Table 2, being most frequent: intraabdominal abscess (8.8%), anastomosis leak (8.8%) and pneumonia (5.4%). Thirty-six patients (24.5%) presented one POC, 18 (12.2%) patients had 2 and 6 (4%) patients had ≥ 3 POC.
Patients with high preoperative NLR (p = 0.69) did not present more POC. The postoperative mortality rate at 30 days was 6.1%.
Preoperative NLR results
Median preoperative leukocyte counts and ratios were neutrophils 4.2 × 109/L (1.6–14.2), lymphocytes 1.8 × 109/L (0.4–3.9) and NLR 2.4 (0.8–16.4). The 50th percentile of NLR (2.4) was used as a cut-off value. The sample was divided into two groups according to NLR value. Both groups had similar clinical characteristics, histological types and tumour stage, but infectious POC were more prevalent in the NLR ≥ 2.4 group (p < 0.001) (Table 1).
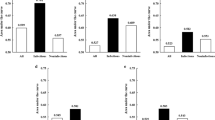
Patients with POC showed worse long-term survival (p = 0.000) (Fig. 2), with no difference (p = 0.867) between infectious (29.1 months [4.10–54.13], 5-year overall survival 21.3 months) or non-infectious POCs (29.9 months [17.18–42.62], 5-year overall survival 25.6 months).
The group of patients with preoperative NLR ≥ 2.4 (p = 0.02) also had a worse prognosis (Fig. 3). NLR maintained its prognostic relevance (p = 0.00) in patients with NLR < and ≥ 2.4, with and without POC (Fig. 4A, B). Similarly, NLR was also related to survival in the group of patients with stage III tumours (34.04 months [95% CI 19.05–49.02] vs. 15.37 months [95% CI 12.44–18.31], p = 0.01).
In the univariant survival analysis, factors related to worse long-term survival were the type of surgery, D2 lymphadenectomy, serosa invasion (pT3-4), nodal metastasis, the existence of POC (especially those classified as Clavien–Dindo ≥ 3) and preoperative NLR ≥ 2.4 (Table 3).
Multivariant analysis showed that lymph node metastases (pN) (p < 0.001), presence of postoperative complications (p < 0.001) and NRL ≥ 2.4 (p = 0.04) were independently associated with poor survival (Table 3).
Discussion
This study shows that the preoperative systemic inflammatory response of the host correlates with overall survival independently in patients with curative gastric cancer resection. Also, postoperative complications and their severity were associated with the worst prognosis, but the inflammatory response of postoperative complications (which differs from the inflammation produced by the oncological process) did not affect preoperative NLR.
The prognostic value of preoperative NLR has previously been reported for gastric cancer and other types of tumours [2, 4, 21,22,23,24,25,26,27]. It is well known that peripheral blood neutrophils, lymphocytes, monocytes and platelets, as well as their combination by ratios, behave as inflammatory markers in patients with cancer and play a relevant role in tumour-related immunity. However, the relationship between blood levels and the local inflammatory tumour microenvironment has not been established to date.
Neutrophils could promote growth and metastasis of tumours through secreting cytokines, chemokines and vascular endothelial growth factor and promote adhesion between circulating tumoral cells and distant organs, increasing the chance of distant metastases [28]. Moreover, neutrophils could also inhibit the antitumour immune function of the Natural Killer and cytotoxic T cells. Lymphocytes play a relevant role in tumour-related immunity. Several subtypes of tumour infiltrating lymphocytes such as CD8 + T cells and memory T cells are associated with better outcomes, but some subsets of T cells, regulatory T cells and Th17 cells are related to tumour progression and unfavourable prognosis. However, a high level of absolute lymphocytes count in blood is associated with an antitumour function, inhibition of tumour progression and favourable prognosis [29].
This study showed that pT, pN, type of surgery, type of lymphadenectomy, NLR and severity of postoperative complications (C-D) were predictors of long-term survival. However, multivariate analysis showed that only pN, postoperative complications and NLR remained independent prognostic factors. These findings underline that systemic inflammatory status has an important influence on the prognosis of patients with gastric cancer, independently from tumour stage and the presence of POC, suggesting that NLR can behave as a reliable marker of the host inflammatory status against the tumour.
However, it has been described [30, 31] that an altered preoperative inflammatory state of the patient can favour POC and these POC (especially the infectious and severe ones) are related to a higher risk of tumour recurrence. It could be argued that only morbidity itself has a real influence on long-term prognosis rather than the preoperative systemic inflammatory state [10, 11, 30].
Our results suggest that the mechanisms through which preoperative systemic inflammatory response (NLR ≥ 2.4) influences overall survival were not mediated through the development of surgical complications. Both parameters were independent prognostic factors. This is an important concept because POC are not uncommon after gastric cancer surgery and affect almost 40% of patients in this study, the most common being anastomotic leaks (8.8%) and intraabdominal abscess (8.8%). These results are in concordance with recent studies [32].
Our results also show the importance of the preoperative value of NLR in the prognosis and its influence on the overall survival of different anatomopathological stages (TNM classification). NLR significantly influenced stages III and IV so that patients with the same TNM stage had different overall survival according to NLR value. This phenomenon could be explained because most of the patients of our cohort had an advanced TNM stage. This is an important observation because it allows us to differentiate those patients with the same TNM stage and different long-term survival [33, 34].
It is known that surgery and non-infectious complications (like obstruction, perforation and haemorrhage) are associated with the generation of systemic inflammatory response, resulting in the suppression of cell-mediated immunity [35]. The development of postoperative infectious complications results in an up-regulation of innate immune response and the suppression of adaptive immunity, favouring an increased risk of recurrence [30].
Previous hypotheses relating to surgical complications and a reduction in survival were based on the paradigm that infective complications initiate an inflammatory cascade, activate pro-inflammatory cytokines and vascular growth factors which promote tumour growth and dissemination [36]. Although the mechanisms are not well known, the systemic inflammatory response induced by the tumour may differ from that induced by surgical trauma or infection. An elevated preoperative NLR has been associated with a greater density of CD4 + lymphocytes around the tumour, without other specific immune cells, like CD3 + or CD8 + lymphocytes, suggesting a relationship between peripheral inflammatory response and immune activation in the local tumour microenvironment [37]. In our series, we found more infectious POC in the NLR ≥ 2.4, in line with previous studies [31, 38], but this phenomenon did not correlate with differences in overall survival.
Taking all these reasons into account, we should differentiate preoperative tumour-related inflammation from the inflammatory mechanisms of POC, which will influence prognosis in different ways.
Another important issue in most studies is the lack of consensus on the cut-off value for NLR. One of the controversies of this type of study is the lack of standardisation to determine the cut-off value for NLR, ranging in the literature from 2 to 5 [33, 39,40,41]. We used an NLR cut-off value of 2.4, based on the median value, with similar results to other studies [9, 42]. However, different methods can be used to calculate NLR, such as ROC curve, median value or the use of computer X-Tile software [29]. Because of this variability in the cut-off values, it is difficult to use preoperative NLR as a clinical standardised prognostic value. Besides, a combination of different preoperative systemic inflammatory markers (NLR, LMR, total number of monocytes and lymphocytes) may provide a better predictive value than each one alone, but this has not been confirmed to date [43].
The limitations of this study include those related to its retrospective design, the limited number of patients, the impossibility of having a standard cut-off value for NLR and that the prognostic value of peripheral blood cells after surgery was not evaluated. More studies are needed to establish a clear cut-off value of preoperative inflammatory markers and help to find its utility in clinical practice.
In conclusion, the preoperative systemic inflammatory response in patients with gastric cancer, measured by neutrophil-to-lymphocyte ratio, behaves as an independent prognostic factor, even in those patients with postoperative complications. More prospective trials are necessary to validate these data.
Data Availability
Not applicable.
Code availability
Not applicable.
References
Ostrand-Rosenberg S (2008) Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev 18:11–18. https://doi.org/10.1016/j.gde.2007.12.007
Guthrie GJK, Charles KA, Roxburgh CSD et al (2013) The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 88:218–230. https://doi.org/10.1016/j.critrevonc.2013.03.010
Lieto E, Galizia G, Auricchio A et al (2017) Preoperative neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio are prognostic factors in gastric cancers undergoing surgery. J Gastrointest Surg 21:1764–1774. https://doi.org/10.1007/s11605-017-3515-x
Lian L, Xia YY, Zhou C et al (2015) Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark 15:899–907. https://doi.org/10.3233/CBM-150534
Nozoe T, Ninomiya M, Maeda T et al (2010) Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today 40:440–443. https://doi.org/10.1007/s00595-009-4065-y
Proctor MJ, Morrison DS, Talwar D et al (2011) A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 47:2633–41. https://doi.org/10.1016/j.ejca.2011.03.028
Feng JF, Chen S, Yang X (2017) Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Med (United States) 96:5. https://doi.org/10.1097/MD.0000000000005886
Song S, Li C, Li S et al (2017) Derived neutrophil to lymphocyte ratio and monocyte to lymphocyte ratio may be better biomarkers for predicting overall survival of patients with advanced gastric cancer. Onco Targets Ther 10:3145–3154. https://doi.org/10.2147/OTT.S138039
Wang SC, Chou JF, Strong VE et al (2016) Pretreatment neutrophil to lymphocyte ratio independently predicts disease-specific survival in resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg 263:292–297. https://doi.org/10.1097/SLA.0000000000001189
Li Z, Bai B, Ji G et al (2018) Relationship between Clavien-Dindo classification and long-term survival outcomes after curative resection for gastric cancer: a propensity score-matched analysis. Int J Surg 60:67–73. https://doi.org/10.1016/j.ijsu.2018.10.044
Li Z, Bai B, Zhao Y et al (2018) Severity of complications and long-term survival after laparoscopic total gastrectomy with D2 lymph node dissection for advanced gastric cancer: a propensity score-matched, case–control study. Int J Surg 54:62–69. https://doi.org/10.1016/j.ijsu.2018.04.034
Lerut T, Moons J, Coosemans W et al (2009) Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 250:798–806. https://doi.org/10.1097/SLA.0b013e3181bdd5a8
Roxburgh CS, Horgan PG, McMillan DC (2013) The perioperative immune/inflammatory insult in cancer surgery: time for intervention? Oncoimmunology 2:11–13. https://doi.org/10.4161/onci.27324
Kodera Y, Sano T (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 20:1–19. https://doi.org/10.1007/s10120-016-0622-4
Wittekind C, Sobin L GM. TNM: classification of malignant tumours. TNM Classification of Malignant Tumours, 7th edn. 2009. 1–310 p
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 64:31–49. https://doi.org/10.1111/apm.1965.64.1.31
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Climent M, Hidalgo N, Vidal Ó et al (2016) Postoperative complications do not impact on recurrence and survival after curative resection of gastric cancer. Eur J Surg Oncol 42:132–139. https://doi.org/10.1016/j.ejso.2015.08.163
Care S, Series B. Available online at NCCN.org/patients Prostate Cancer NCCN Guidelines for Patients ®. 2016
Kleinberg LR, Md; W. Michael Korn MD. (2016) Clinical practice guidelines in oncology NCCN categories of evidence and consensus. JNCCN-Journal Natl Compr Cancer Netw. 14:1286–312
Aizawa M, Gotohda N, Takahashi S, T. et al (2011) Predictive value of baseline neutrophil/lymphocyte ratio for T4 disease in wall-penetrating gastric cancer. World J Surg. 35:2717–22. https://doi.org/10.1007/s00268-011-1269-2
Mano Y, Shirabe K, Yamashita Y et al (2013) Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma. Ann Surg 258:301–305. https://doi.org/10.1097/SLA.0b013e318297ad6b
Williams KA, Labidi-Galy SI, Terry KL et al (2014) Prognostic significance and predictors of the neutrophil-to-lymphocyte ratio in ovarian cancer. Gynecol Oncol 132:542–550. https://doi.org/10.1016/j.ygyno.2014.01.026
Yang J-J, Hu Z-G, Shi W-X et al (2015) Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta-analysis. World J Gastroenterol 21:2807–2815. https://doi.org/10.3748/wjg.v21.i9.2807
Jiang C, Hu W-M, Liao F-X et al (2016) Elevated preoperative neutrophil-to-lymphocyte ratio is associated with poor prognosis in gastrointestinal stromal tumor patients. Onco Targets Ther 9:877–883. https://doi.org/10.2147/OTT.S90569
Tang H, Ma H, Peng F et al (2016) Prognostic performance of inflammation-based prognostic indices in locally advanced non-small-lung cancer treated with endostar and concurrent chemoradiotherapy. Mol Clin Oncol 4:801–806. https://doi.org/10.3892/mco.2016.796
Kim HS, Ku JH (2016) Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers 2016:1–12. https://doi.org/10.1155/2016/8345286
Gonzalez H, Hagerling C, Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32:1267–1284. https://doi.org/10.1101/gad.314617.118
Feng F, Zheng G, Wang Q et al (2018) Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol 18:1–7. https://doi.org/10.1186/s12876-018-0877-9
Mohri Y, Tanaka K, Toiyama Y et al (2016) Impact of preoperative neutrophil to lymphocyte ratio and postoperative infectious complications on survival after curative gastrectomy for gastric cancer: a single institutional cohort study. Medicine (Baltimore) 95:e3125. https://doi.org/10.1097/MD.0000000000003125
Mohri Y, Miki C, Kobayashi M et al (2014) Correlation between preoperative systemic inflammation and postoperative infection in patients with gastrointestinal cancer: a multicenter study. Surg Today 44:859–867. https://doi.org/10.1007/s00595-013-0622-5
van der Werf LR, Busweiler LAD, van Sandick JW et al (2020) Dutch Upper GI Cancer Audit (DUCA) group. Reporting national outcomes after esophagectomy and gastrectomy according to the Esophageal Complications Consensus Group (ECCG). Ann Surg. 271:1095–1101. https://doi.org/10.1097/SLA.0000000000003210
Conway AM, Salih Z, Papaxoinis G, et al. Significance of blood neutrophil-to-lymphocyte ratio for prognostic stratification of patients with gastroesophageal junction adenocarcinoma in the era of the 8th edition of the American Joint Committee on Cancer (AJCC8) staging. Med Oncol. 2017;34. https://doi.org/10.1007/s12032-017-0976-4.
Jung MR, Park YK, Jeong O et al (2011) Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol 104:504–510. https://doi.org/10.1002/jso.21986
Marik PE, Flemmer M (2012) The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg 73:801–808. https://doi.org/10.1097/TA.0b013e318265cf87
Richards CH, Platt JJ, Anderson JH et al (2011) The impact of perioperative risk, tumor pathology and surgical complications on disease recurrence following potentially curative resection of colorectal cancer. Ann Surg 254:83–89. https://doi.org/10.1097/SLA.0b013e31821fd469
Gutkin DW, Shurin MR (2014) Clinical evaluation of systemic and local immune responses in cancer: time for integration. Cancer Immunol Immunother 63:45–57. https://doi.org/10.1007/s00262-013-1480-0
Moyes LH, Leitch EF, McKee RF et al (2009) Preoperative systemic inflammation predicts postoperative infectious complications in patients undergoing curative resection for colorectal cancer. Br J Cancer 100:1236–1239. https://doi.org/10.1038/sj.bjc.6604997
Lin W, Karin M, Lin W, Karin M (2007) A cytokine-mediated link between innate immunity, inflammation, and cancer find the latest version : review series a cytokine-mediated link between innate immunity, inflammation, and cancer 117:1175–1183. https://doi.org/10.1172/JCI31537
Sun J, Chen X, Gao P et al (2016) Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? A systematic review and meta-analysis. Dis Markers 2016:7862462. https://doi.org/10.1155/2016/7862469
Mellor KL, Powell AGMT, Lewis WG (2018) Systematic review and meta-analysis of the prognostic significance of neutrophil-lymphocyte ratio (NLR) after R0 gastrectomy for cancer. J Gastrointest Cancer 49:237–244. https://doi.org/10.1007/s12029-018-0127-y
Szor DJ, Roncon-Dias A, Pereira M et al (2018) Neutrophil-lymphocyte ratio is associated with prognosis in patients who underwent potentially curative resection for gastric cancer. J Surg Oncol 11:851–857. https://doi.org/10.1002/jso.25036
Eo WK, Jeong DW, Chang HJ et al (2015) Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol 21:2668–2676. https://doi.org/10.3748/wjg.v21.i9.2668
Acknowledgements
We want to especially thank Sylva-Astrik Torossian for her assistance in language editing. This study is part of the doctoral programme of the Department of Surgery at Universitat Autònoma de Barcelona.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona.
Author information
Authors and Affiliations
Contributions
J. Tur-Martínez and N. Puértolas-Rico collected data. J. Tur-Martínez and J. Rodríguez-Santiago designed the study, performed statistical analysis and drafted the manuscript. M. Pera, J. Osorio, N. Pérez-Romero, N. Puértolas-Rico and S. Delgado revised the manuscript critically and made substantial contribution. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The present study was evaluated and approved by the Ethical Committee of our hospital.
Consent to participate
Because of the retrospective character of the study, non-informed consent was required. The study was approved by the ethical committee of our hospital (Acta 10/2019).
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tur-Martínez, J., Osorio, J., Pérez-Romero, N. et al. Preoperative neutrophil-to-lymphocyte ratio behaves as an independent prognostic factor even in patients with postoperative complications after curative resection for gastric cancer. Langenbecks Arch Surg 407, 1017–1026 (2022). https://doi.org/10.1007/s00423-022-02432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-022-02432-9